I-Mab Unveils Promising Phase 1 Results for Ragistomig at ASCO 2024
I-Mab, a global biotechnology firm headquartered in the United States, which specializes in the innovation and potential market launch of distinct immunotherapies designed to treat cancer, revealed that Dr. Gerald Falchook and the I-Mab’s collaborator for ragistomig (also known as "ABL503"), ABL Bio, will showcase a poster featuring Phase 1 data for ragistomig at the 2024 American Society for Clinical Oncology Annual Meeting. This event is scheduled to be held from May 31st to June 4th at the McCormick Place Convention Center in Chicago, Illinois.
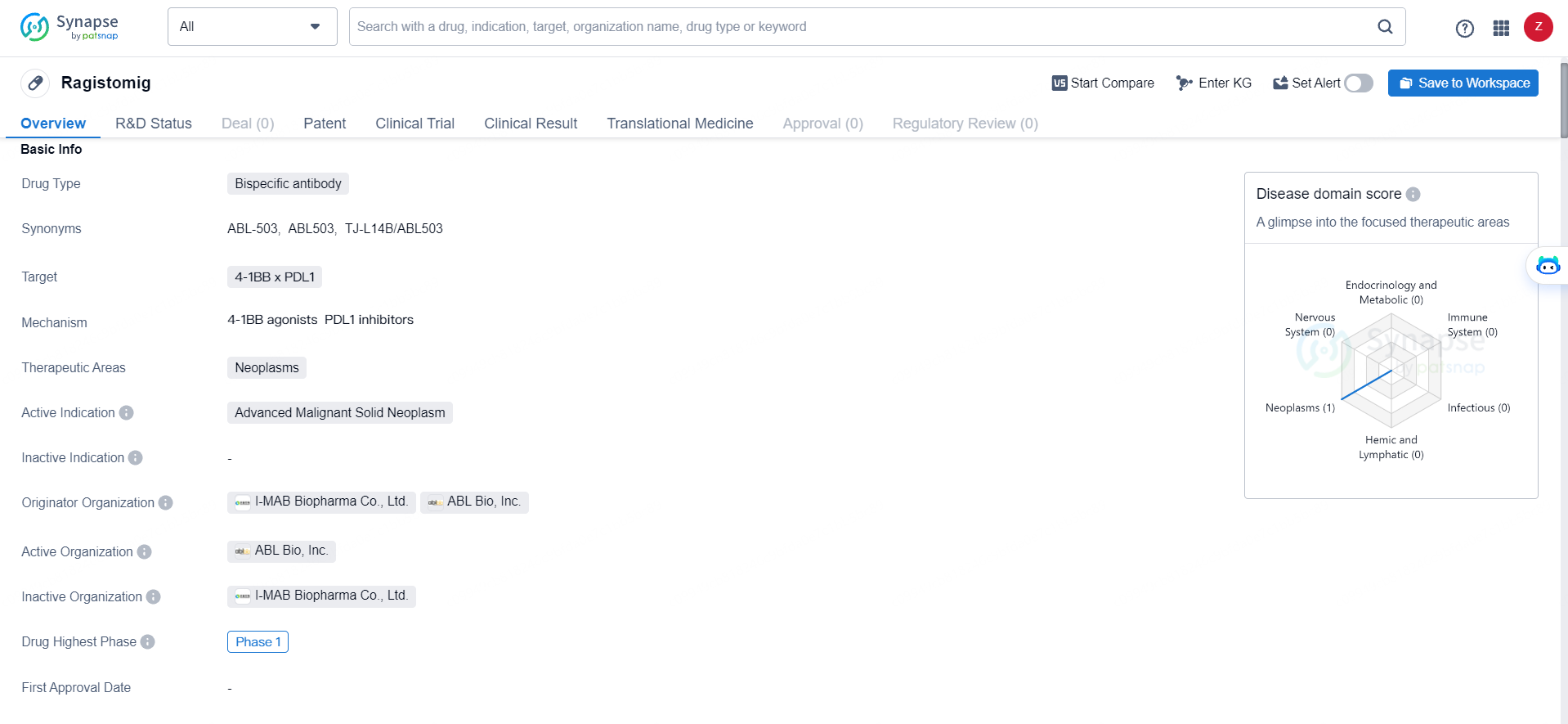
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Ragistomig was developed as a bispecific antibody designed to integrate anti-PD-L1 functionality with 4-1BB-driven T-cell activation within a single molecule. The strategy incorporated an Fc-silent and conditional 4-1BB engagement aimed at enhancing safety, particularly by potentially reducing hepatotoxicity when compared to conventional 4-1BB agonists.
"We are excited to present the latest Phase 1 data for ragistomig at ASCO 2024. Although immune checkpoint inhibitors have greatly advanced the treatment of solid tumors, a number of tumors either do not respond or eventually become resistant to these agents. Ragistomig aims to offer a novel therapeutic option for patients who are resistant to immune checkpoint inhibitors," stated Raj Kannan, Chief Executive Officer at I-Mab.
"Initial data suggest that the study has achieved its goals, allowing us to define an optimal dosage and showing early signs of efficacy in patients who have relapsed or are refractory to previous checkpoint inhibitor treatments. These findings further support the ongoing development of ragistomig not only as a monotherapy but also in combination with other treatments. We are eager to progress the clinical program in partnership with ABL Bio," Raj Kannan added.
Jointly developed with ABL Bio, ragistomig (also referred to as ABL503) is a unique bispecific antibody targeting PD-L1. It combines an Fc-silent anti-PD-L1 arm, which acts as the tumor-dependent T-cell activator, with a single chain variable fragment of an anti-4-1BB engaging antibody, serving as the conditional T-cell activator in the presence of tumor engagement.
Leveraging ABL Bio's "Grabody-T" bispecific antibody platform technology, ragistomig/ABL503 specifically activates 4-1BB only when PD-L1 expressing tumor cells are present, thereby minimizing the risk of off-tumor toxicity and addressing resistance to PD-(L)1 inhibition. Preclinical studies have shown that the bispecific antibody exhibits superior anti-tumor activity compared to equimolar doses of single agents, whether used alone or in combination..
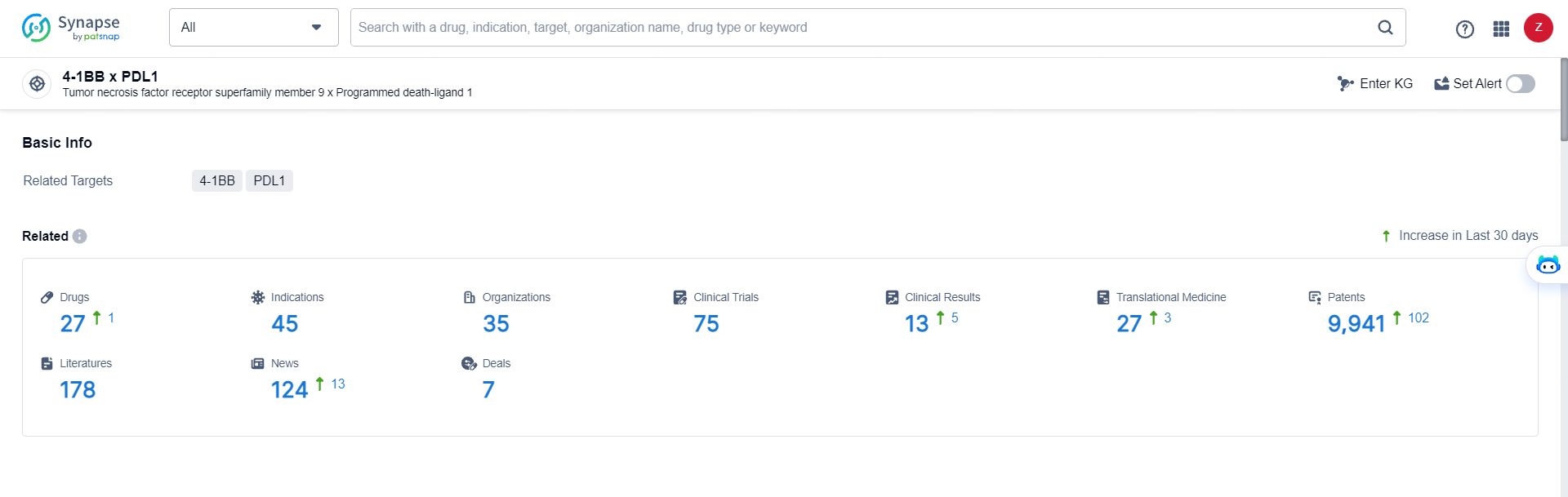
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 29, 2024, there are 27 investigational drugs for the 4-1BB x PDL1 targets, including 45 indications, 35 R&D institutions involved, with related clinical trials reaching 75, and as many as 9941 patents.
Ragistomig is a bispecific antibody drug that targets 4-1BB x PDL1 and is primarily focused on the therapeutic areas of neoplasms. Ragistomig represents a promising development in the field of biomedicine, particularly in the area of cancer immunotherapy. As the drug progresses through clinical development, further insights into its potential benefits and limitations will likely emerge, ultimately shaping its future role in the treatment of advanced malignant solid neoplasms.