Sensei Biotherapeutics Shares Encouraging Phase 1 Dose Escalation Study Results for SNS-101
Sensei Biotherapeutics, Inc., an immuno-oncology company in the clinical stage that concentrates on discovering and developing advanced therapeutics for cancer treatment, has announced promising clinical results from the dose-escalation segment of its Phase 1/2 study on SNS-101. SNS-101 is a conditionally active, human monoclonal antibody designed to target the immune checkpoint VISTA.
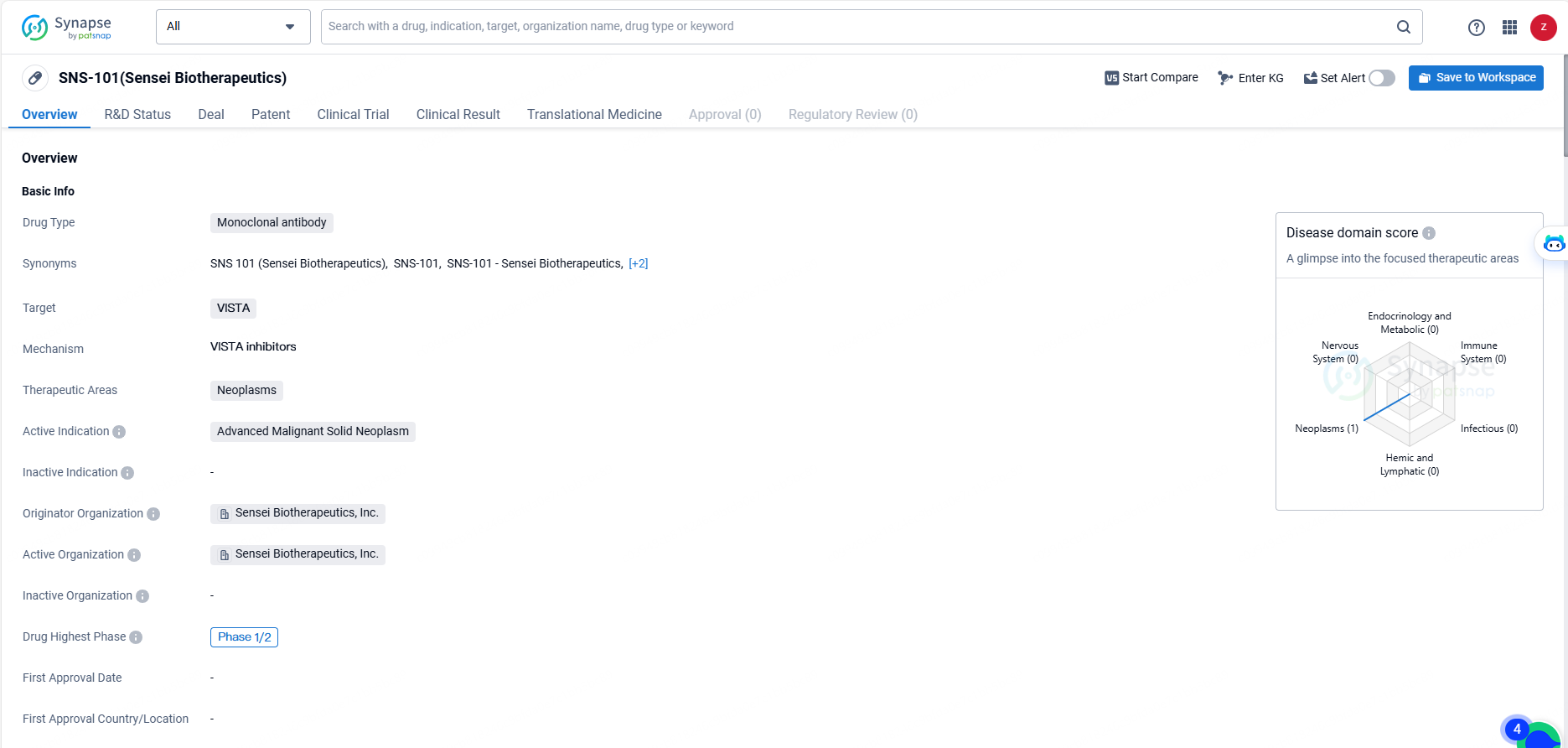
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"I am delighted with the tolerance observed for SNS-101 in this study. It has achieved the highest planned dose with no dose-limiting toxicities, showing promise in addressing previous challenges with VISTA-targeted therapies," stated Dr. Shiraj Sen, Medical Oncologist, Director of Clinical Research at NEXT Oncology, Dallas, and primary investigator for the SNS-101 trial.
"The results reveal promising clinical activity in a varied patient group with advanced solid tumors, especially where clinical responses are typically unexpected, such as in microsatellite stable colorectal and endometrial cancers. There is a persistent unmet need for patients who have developed resistance or do not respond to current immunotherapy treatments. I am eager to continue monitoring its development," Dr. Shiraj Sen further commented.
In the Phase 1/2 clinical trial, the dose-escalation phase is set to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of SNS-101. This study investigates SNS-101 as a standalone treatment and alongside Regeneron’s PD-1 inhibitor Libtayo for patients with advanced solid tumors resistant to initial or acquired PD-1 therapy.
A total of 34 patients received SNS-101 every three weeks, with 16 patients in the monotherapy group and 18 in the combination arm. Most participants had tumor types generally unresponsive to PD-1 monotherapy.
SNS-101 was well tolerated both by itself and with cemiplimab, with no dose-limiting toxicities. Most adverse events were Grade 1 or 2 in severity. Two patients at the highest dose of SNS-101 experienced mild and manageable Grade 1 cytokine release syndrome, one in monotherapy and the other in combination therapy, showcasing SNS-101's potential to resolve challenges faced by first-generation VISTA-targeting therapies. Four patients in the combination cohort showed immune-mediated events. No significant changes were observed in the levels of key inflammatory cytokines, including interferon gamma, interleukin-6, interleukin-10, interleukin-8, TNF-alpha, and CCL5-RANTES, across various groups.
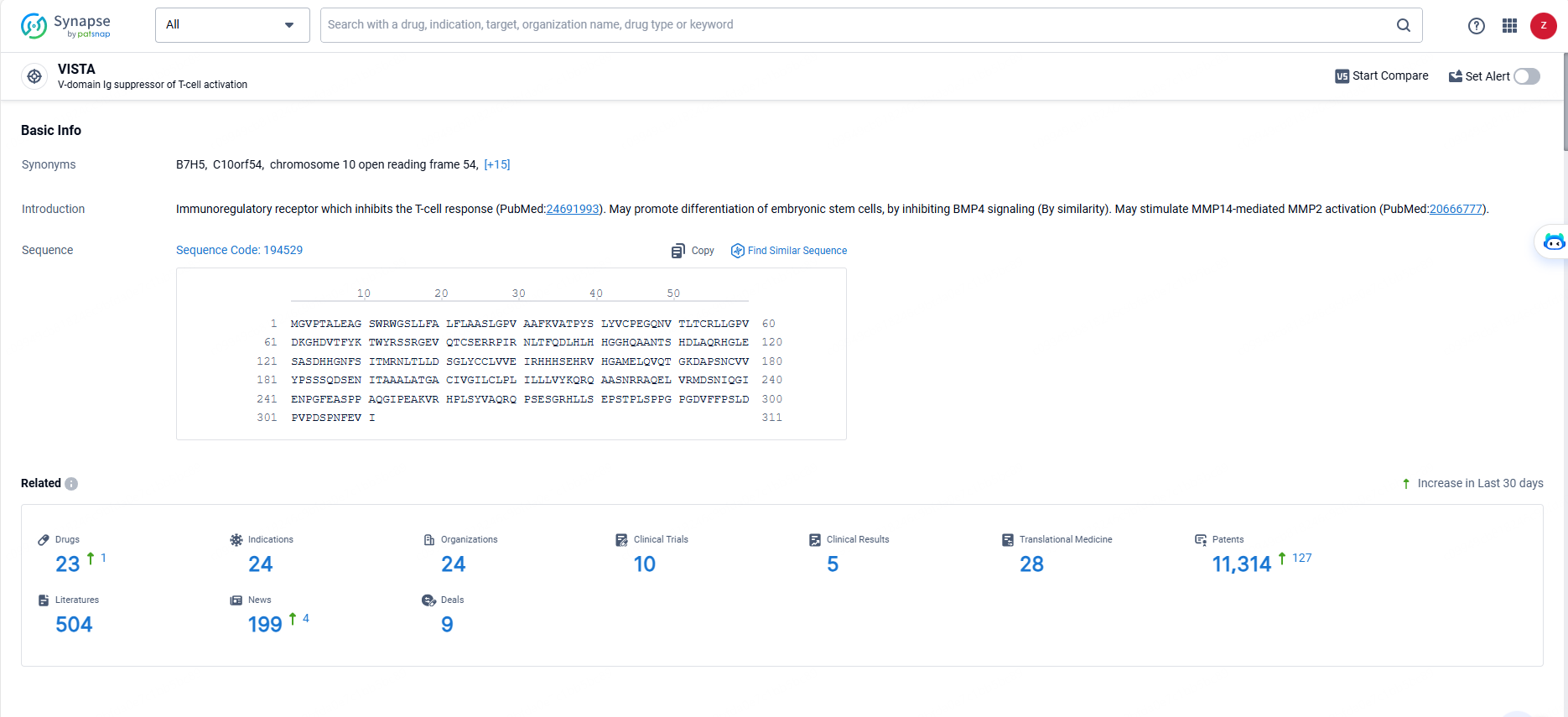
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 29, 2024, there are 23 investigational drugs for the VISTA targets, including 24 indications, 24 R&D institutions involved, with related clinical trials reaching 10, and as many as 11314 patents.
SNS-101 is a monoclonal antibody drug targeting VISTA, being developed by Sensei Biotherapeutics, Inc. for the treatment of advanced malignant solid neoplasms. The drug has reached the Phase 1/2 stage of clinical development, indicating promising early results and potential for further advancement in clinical trials. As with any investigational drug, further research and development are necessary to fully understand its safety and efficacy profile.