Immatics TCR-T therapy IMA203 is granted RMAT designation by CBER for the treatment of multiple types of cancer
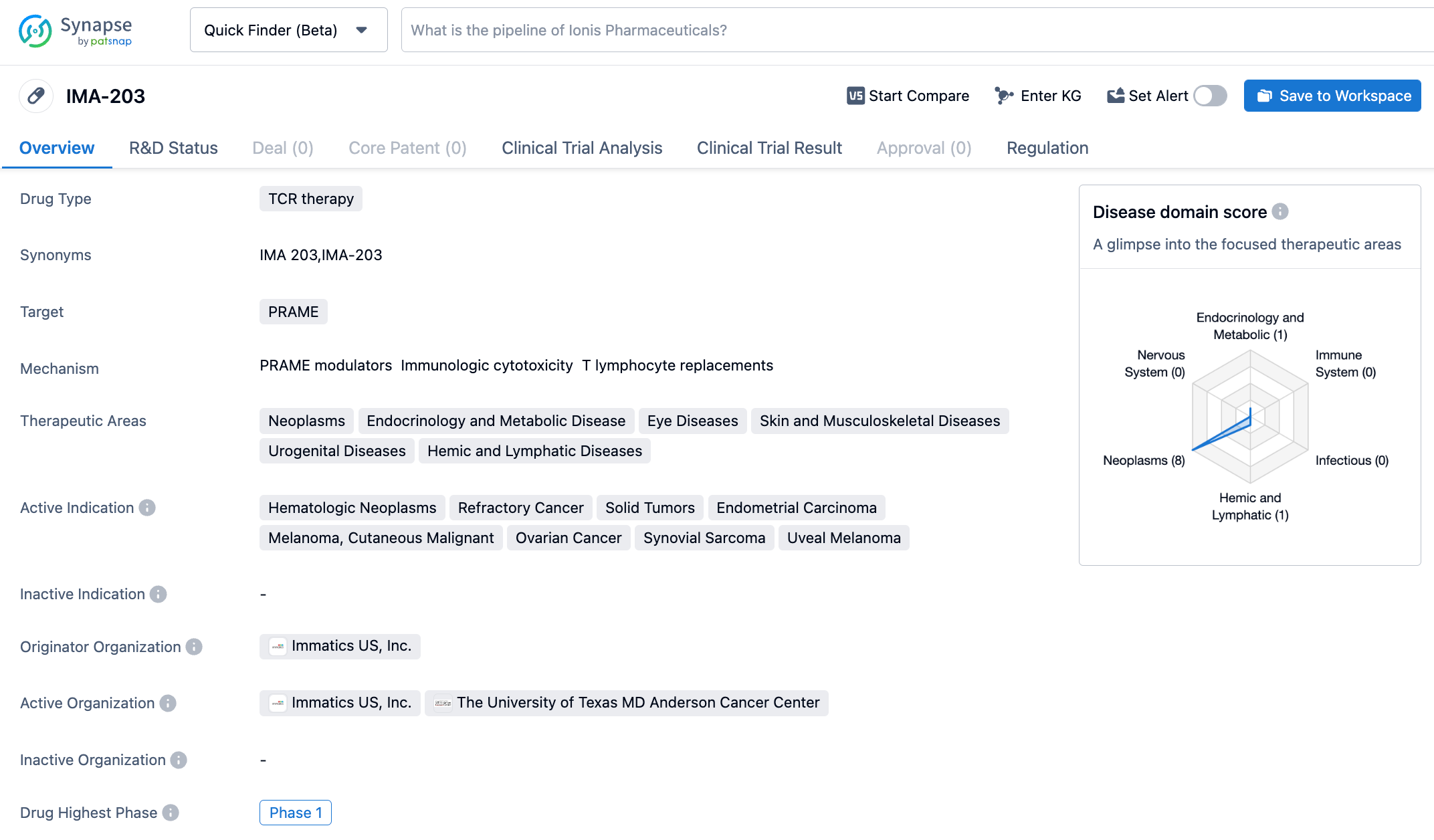
Recently, Immatics announced that its T cell receptor T cell (TCR-T) therapy IMA203 under research has been granted the Regenerative Medicine Advanced Therapy (RMAT) designation by the Center for Biologics Evaluation and Research (CBER) of the U.S. FDA for the treatment of multiple recurrent and/or refractory HLA-A*02:01 positive and PRAME expressed cancers, including melanoma, uveal melanoma, endometrial cancer, synovial sarcoma, and ovarian cancer.
IMA203 is an autologous TCR-T therapy developed on the ACTengine platform of Immatics, which genetically modifies the patient's own T cells to express TCR against the target. In addition, Immatics combined its proprietary cell therapy production platform, capable of completing T cell genetic engineering modification and production within 6-10 days. The therapy targets the melanoma antigen PRAME presented by HLA-A*02. PRAME is a protein often expressed in various solid tumors, and thus IMA203 has the potential to treat a broad spectrum of cancer patients.
In the phase 1b dose extension trial, the median follow-up time was 8.5 months at the cut-off date, the data has not yet reached the median duration of response, the initial objective response rate (ORR) reached 64% (7/11) at the 6th week, and the confirmed ORR was 67% (6/9) at the 3rd month. Patients who achieved objective remission included those with low, medium, and high PRAME expression levels, such as melanoma resistant to checkpoint inhibitors, platinum-resistant ovarian cancer, uveal melanoma, head and neck cancer, and synovial sarcoma. Cohort A patients continued to show controllable tolerability during IMA203 treatment, without observed high grade cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), and no dose-dependent increase in CRS was observed.
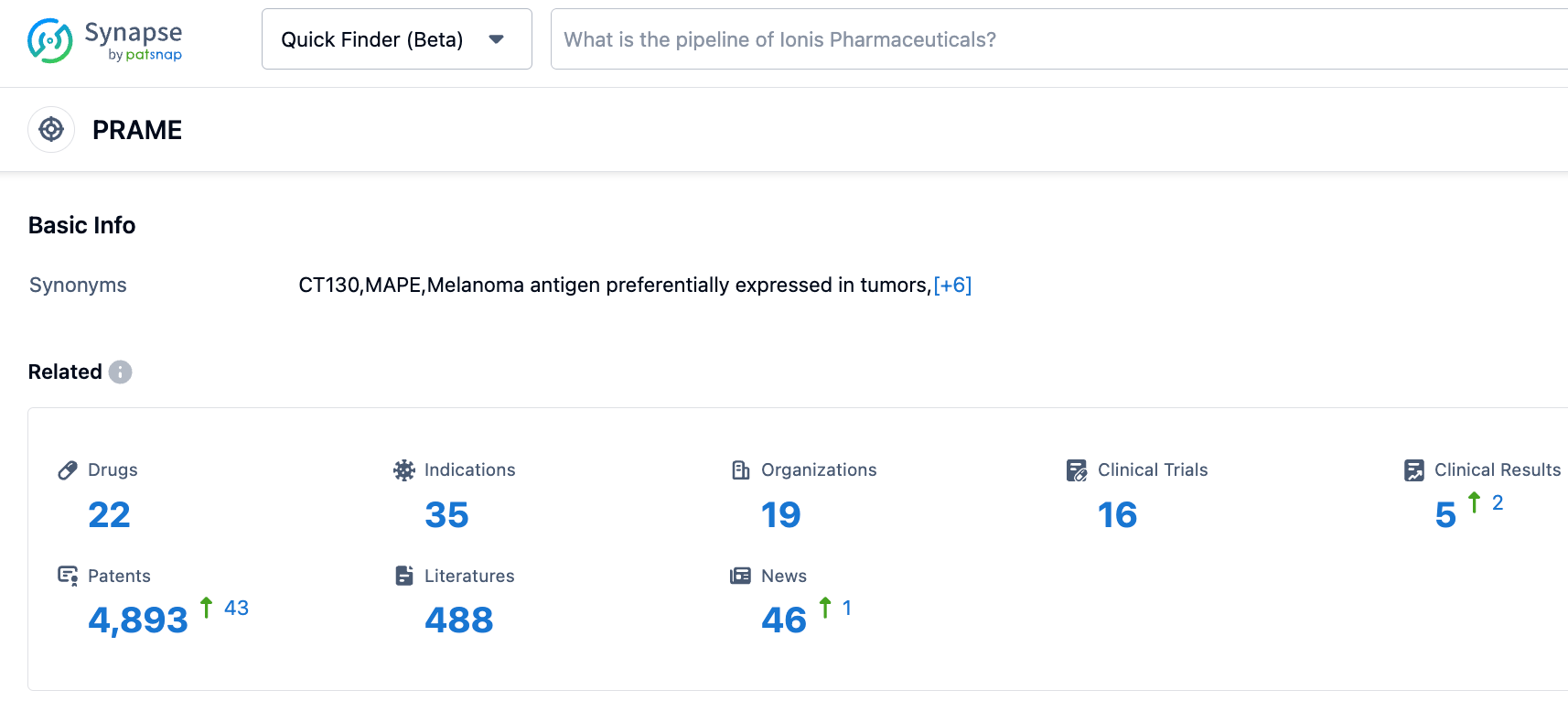
According to the information disclosed by the synapse database, as of October 27, 2023, there were 22 drugs in research with PRAME as the target, encompassing 32 indications, 19 research institutions, 16 related clinical trials, and up to 4891 patents... This is the first time the U.S. FDA has designated an RMAT for a tumor candidate therapy for more than two solid tumor indications. It is hoped that IMA203 can come to market as soon as possible.