ImmunoGenesis administers first patient in the Phase 1a/1b clinical study of IMGS-001 for advanced solid tumors
ImmunoGenesis, a cutting-edge biotech firm focused on developing scientifically backed immunotherapies, has publicized the initial patient treatment within their Phase 1a/1b clinical study employing their primary experimental drug, IMGS-001, at The University of Texas MD Anderson Cancer Center.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
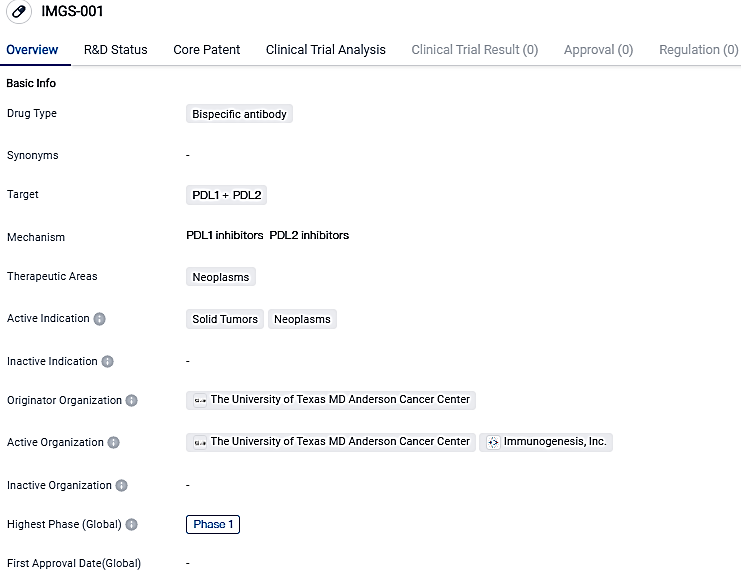
IMGS-001 is a designed dual-specific antibody for programmed cell death 1 ligand 1 (PD-L1)/programmed cell death 1 ligand 2 (PD-L2) possessing cytotoxic properties, intended for the treatment of cold, immune-excluded tumors resistant to available immunotherapies.
The initial Phase 1a/1b study, which is open to public and multi-centered, consists of an escalation and expansion phase to assess safety, pharmacokinetics and preliminary anti-tumor effects of IMGS-001 in adult patients with solid tumors, either locally advanced or metastatic, that have not responded to standard-of-care treatments. Tumor types expected in the escalation phase of the study include ovarian, colorectal and triple-negative breast cancer.
"There's a number of tumors that remain unresponsive to present immunotherapies, which is an area in desperate need of attention," assured ImmunoGenesis President and CEO James Barlow. "Our mission is to widen the patient base that can benefit from immunotherapy by focusing on key mechanisms leading to immune resistance. We are hopeful that this trial will offer an initial proof of concept supporting our innovative approach which employs a single molecule to mitigate immunosuppression and PD-1 pathway blockade."
"PD-L1 and PD-L2 are prolifically expressed not only in various tumors but also in immunosuppressive cells within the tumor microenvironment," disclosed ImmunoGenesis Acting Chief Medical Officer Dr. Jeremy Barton. "IMGS-001 has been engineered to eliminate these immunosuppressive cells and potentially enhance the PD-1 pathway blockade. The initiation of this Phase 1a/1b clinical trial is a significant step towards verifying this approach's efficacy for treating cold, immune-excluded cancers."
The lead initiative at ImmunoGenesis, IMGS-001, is a dual-specific monoclonal antibody focused on PD-L1/PD-L2, possessing engineered cytotoxic effector functionality. IMGS-001 is the pioneering compound to be clinically tested that targets PD-L2 along with PD-L1, potentially augmenting the blockade of the PD-1 pathway.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
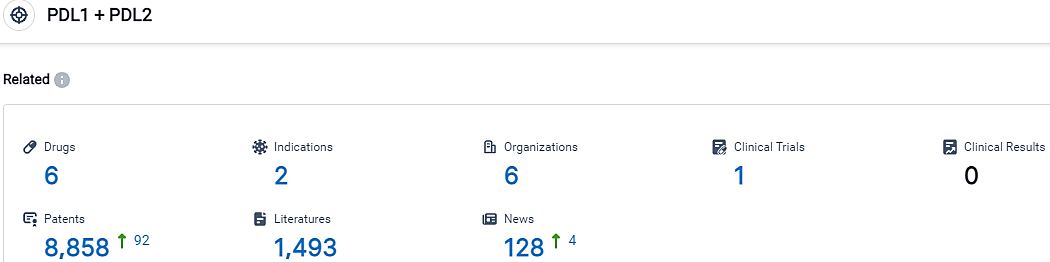
According to the data provided by the Synapse Database, As of October 8, 2023, there are 6 investigational drugs for the PDL1 and PDL2 target, including 2 indications,6 R&D institutions involved, with related clinical trials reaching 1,and as many as 8858 patents.
IMGS-001 is a bispecific antibody drug that targets PDL1 and PDL2 proteins. It is being developed for the treatment of solid tumors and neoplasms. Currently in Phase 1, IMGS-001 shows promise in enhancing the immune response against cancer cells.