In-depth analysis report on breast cancer pipeline drugs
Breast cancer Introduction
Breast cancer is a multifaceted disease that is typified by the unchecked growth of abnormal cells within breast tissue. The disease's development involves numerous factors, including hormonal imbalances, genetic mutations, and environmental factors. Breast cancer usually starts with a small tumor in the breast that can infiltrate nearby tissues and metastasize to distant organs via the bloodstream or lymphatic system. The precise etiology of breast cancer remains unclear, but risk factors like age, family medical history, reproductive elements, and lifestyle decisions play a major role. Early detection and advances in treatment strategies are paramount in battling this disease.
Being the most prevalent type of cancer, breast cancer accounts for 15% of all newly diagnosed cancer cases. In 2022, over 290,000 new incidents are predicted in the United States alone, with an estimated 43,000 fatalities.
This article will highlight the latest list of cancer pipeline drugs, specifically focussed on breast cancer pipeline drugs, pharmaceutical companies of researching breast cancer drugs, drug target of breast cancer, drug type of breast cancer and countries/locations of researching breast cancer drugs.
Breast cancer pipeline drug list
According to Patnsnap Synapse, as of August 15, 2023, there are a total of 2471 Breast Cancer drugs world wide, from 1840 organizations, covering 786 targets, and conducting 18884 clinical trials. To access detailed information regarding breast cancer pipeline drugs, click on the highlighted breast cancer link provided.The top 10 drugs with advanced R&D progress are as follows:
| Drug | Target | Drug Type | Active Organization | Indication | Highest Phase |
| Retifanlimab | PD-1 | Monoclonal antibody | Incyte Corp., MacroGenics, Inc., Zai Lab Ltd. | Metastatic Merkel Cell Carcinoma, Squamous Cell Carcinoma, Endometrial Carcinoma | Approved |
| Omaveloxolone | Nrf2 | Small molecule drug | Reata Pharmaceuticals, Inc. | Friedreich Ataxia, Breast Cancer, Corneal Endothelial Cell Loss | Approved |
| Adebrelimab | PDL1 | Monoclonal antibody | Shanghai Suncadia Pharmaceuticals Co., Ltd., Atridia Pty Ltd., Jiangsu Hengrui Pharmaceuticals Co., Ltd. | ES-SCLC, Non-Small Cell Lung Cancer, HER2 Positive Breast Cancer | Approved |
| Elacestrant | ERα | Small molecule drug | Stemline Therapeutics, Inc., A. Menarini Industrie Farmaceutiche Riunite SRL, Radius Pharmaceuticals, Inc. | ER-positive/HER2-negative/ ESR1-mutated breast cancer, Breast Cancer, Breast Carcinoma Metastatic in the Brain | Approved |
| Mirvetuximab soravtansine | FOLR1 + Tubulin | Monoclonal antibody, Antibody drug conjugate (ADC) | ImmunoGen, Inc., Hangzhou Zhongmeihuadong Pharmaceutical Co., Ltd. | Platinum-Resistant Epithelial Ovarian Carcinoma, Platinum-Resistant Fallopian Tube Carcinoma, Platinum-Resistant Primary Peritoneal Carcinoma | Approved |
| Tremelimumab | CTLA4 | Monoclonal antibody | AstraZeneca PLC, AstraZeneca Global R&D (China) Co., Ltd., MedImmune LLC | Advanced Lung Non-Small Cell Carcinoma, Hepatocellular Carcinoma, Non-Small Cell Lung Cancer | Approved |
| Futibatinib | FGFRs | Small molecule drug | Taiho Pharmaceutical Co., Ltd., Taiho Oncology, Inc. | FGFR2 fusion or rearranged Cholangiocarcinoma, Intrahepatic Cholangiocarcinoma, Biliary Tract Neoplasms | Approved |
| Pucotenlimab | PD-1 | Monoclonal antibody | Akeso Biopharma Co., Ltd., Taizhou Hanzhong biomedical co. LTD, Lepu Biopharma Co., Ltd. | Melanoma, Microsatellite instability-high cancer, Metastatic Colorectal Carcinoma | Approved |
| Serplulimab | PD-1 | Monoclonal antibody | Hengenix Biotech, Inc., Shanghai Henlius Biotech, Inc., Shanghai Fosun Pharmaceutical Industrial Development Co., Ltd. | ES-SCLC, Squamous non-small cell lung cancer, Microsatellite Instability cancer | Approved |
| Icaritin | ERα | Herbal medicine | Lee's Pharmaceutical Holdings Ltd., Beijing Shenogen Biomedical Co., Ltd, Beijing Shenogen Pharmaceutical Co., Ltd | Hepatocellular Carcinoma, Liver Cancer, Neoplasms | Approved |
Pharmaceutical companies of researching breast cancer drugs
Each company has a unique R&D progress, with a focus on different stages of development. Pfizer Inc. has a diverse pipeline, Roche Holding AG is focused on Phase 3 trials, Novartis AG has a balanced distribution across stages, AstraZeneca PLC has a strong presence in Phase 2 trials, and Daiichi Sankyo Co., Ltd. has a smaller but focused pipeline. These companies, along with others in the industry, are actively working towards advancing new treatments for breast cancer.
To access detailed information regarding pharmaceutical companies of breast cancer: First, click on the highlighted link provided for breast cancer. Then, you will see the image below on the page that opens. Click on the numerical figure displayed on that page.

Drug target of breast cancer
Breast cancer treatment has seen significant advancements in the pharmaceutical industry, with various targets being explored for drug development. The targets that have been approved or are under development for breast cancer treatment include HER2, VEGF-A, ER, Tubulin, DNA, AR, DNA + Top II, TYMS, CDK4 + CDK6, Aromatase, and others. These targets provide potential avenues for the development of new and effective drugs to combat breast cancer. Further research and development in these areas can lead to improved treatment options and better outcomes for patients with breast cancer.
To access detailed information regarding drug target of breast cancer : First, click on the highlighted link provided for breast cancer. Then, you will see the image below on the page that opens. Click on the numerical figure displayed on that page.

Drug type of breast cancer
Small molecule drugs, monoclonal antibodies, biosimilars, and diagnostic radiopharmaceuticals show significant progress in breast cancer treatment. The presence of biosimilars indicates intense competition around innovative drugs. Further analysis is required to identify the research and development institutions involved in the production of biosimilars.
To access detailed information regarding drug type of breast cancer : First, click on the highlighted link provided for breast cancer. Then, you will see the image below on the page that opens. Click on the numerical figure displayed on that page.

Countries/locations of researching breast cancer drugs
China, the European Union, and the United States are the leading countries/locations in the development of drugs for Breast Cancer. China, in particular, has shown significant progress with a high number of drugs in all phases. This indicates a strong commitment to advancing Breast Cancer treatments in China.
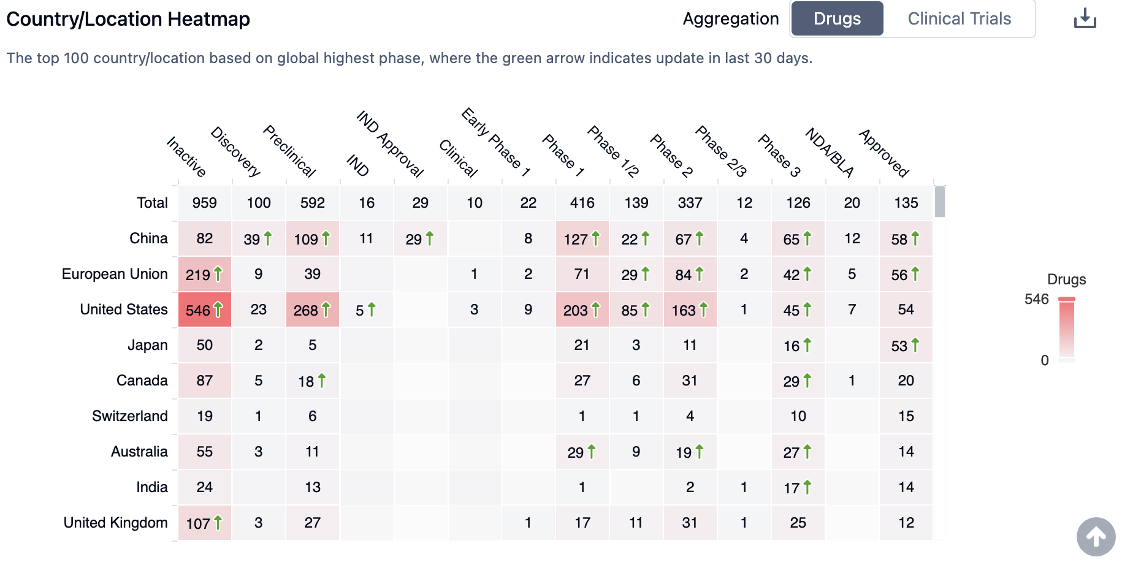
To access detailed information regarding countries/locations of breast cancer: First, click on the highlighted link provided for breast cancer. Then, you will see the image below on the page that opens. Click on the numerical figure displayed on that page.
Conclusion
In conclusion, breast cancer is a multifaceted disease with numerous underlying causal factors. There are several drugs in various stages of development, with 2471 drugs currently on the worldwide breast cancer drug list. Furthermore, the top 10 drugs with advanced R&D progress are approved for various cancer treatments. Prominent pharmaceutical companies, such as Pfizer Inc., Roche Holding AG, Novartis AG etc., are heavily invested in progressing breast cancer treatment. The drug targets for these treatments are diverse, and there is significant development in small molecule drugs, monoclonal antibodies, biosimilars, and diagnostic radiopharmaceuticals. There are marked contributions from various nations towards breast cancer research, with China, European Union, and the United States leading in terms of drug development. To fully grasp the scope and breadth of each topic, direct links are provided for further information.