Dexmedetomidine's Unexpected Rescue of Cognition from Sleep Loss
Insufficient sleep has emerged as an escalating crisis in our society. A 2019 global sleep survey conducted by Philips and KJT Group across 12 countries found 62% of adults don't feel they get adequate sleep, with most not achieving the recommended 8 nightly hours. Over 20% of the general adult population in the U.S. and Canada have reported experiencing insomnia.
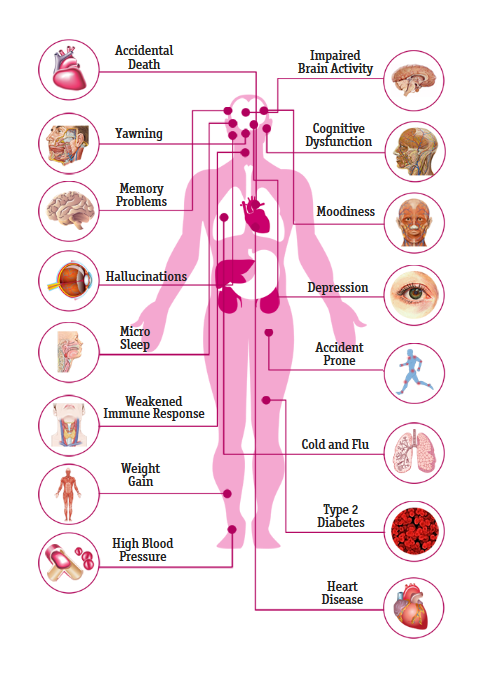
Chronic sleep deprivation can substantially increase morbidity from various diseases including cardiovascular illnesses and neurodegenerative disorders. Studies demonstrate sleep deprivation leads to decreased reaction speed and perceptual ability to external events, but its impacts on other higher-level cognitive abilities remain unclear.
A sedative for sleeplessness
Recently, researchers from Imperial College London published a study titled “Reducing complement activation during sleep deprivation yields cognitive improvement by dexmedetomidine” in the British Journal of Anaesthesia (2023 IF: 9.8). This study first revealed that dexmedetomidine inhibits the microglial synaptic elimination process mediated by the complement C3-C3aR signaling pathway, elucidating a new mechanism by which dexmedetomidine improves cognitive impairment associated with sleep deprivation. This provides new strategies for treating diseases related to sleep disorders.
Dexmedetomidine (DEX) is a novel, highly selective and specific α2 adrenergic receptor agonist that serves as a biomimetic sleep medication without respiratory depression, widely used clinically for sedation of perioperative and ICU patients. Some research indicates dexmedetomidine can effectively reduce incidence of postoperative cognitive dysfunction, but whether this drug ameliorates cognitive impairment from sleep deprivation and its precise mechanisms remain uncertain.
Microglia, the brain's predominant innate immune cells, play a vital role in synaptic pruning and elimination during neural development and synaptic functional shaping. Under pathological conditions, aberrant microglial phagocytosis of synapses mediated by the complement C3-C3aR signaling pathway precipitates cognitive dysfunction.
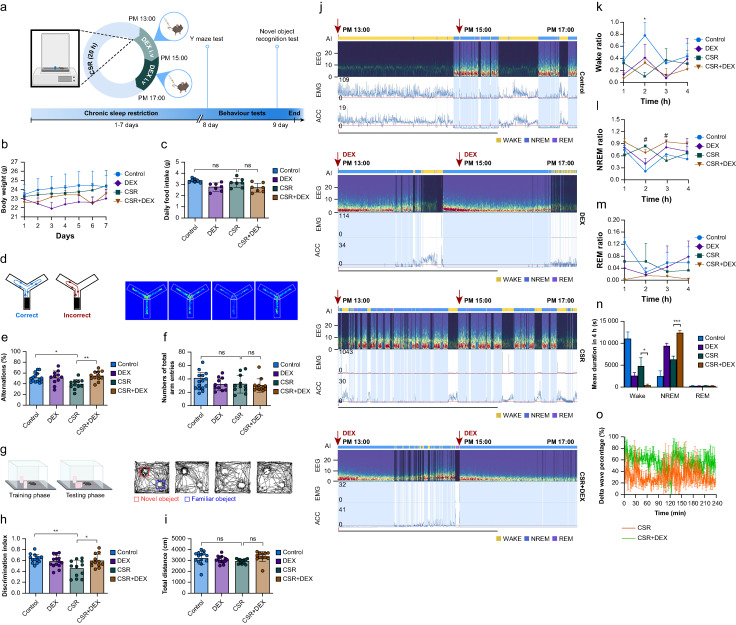
However, whether dexmedetomidine ameliorates cognitive deficits from sleep deprivation by regulating this complement-mediated microglial synaptic pruning remains unknown. To illuminate these scientific mysteries, the researchers first developed a chronic sleep restriction mouse model, discovering these mice displayed impaired learning and memory while intravenous dexmedetomidine improved cognitive dysfunction in sleep-deprived mice. Along with electroencephalographic data, this compellingly demonstrates dexmedetomidine can strengthen sleep quality and remedy cognitive impairments from sleep loss.
Synapses form the substrate for encoding and storing memories, with alterations in synapse number and function impacting cognition. Examining mouse hippocampal tissues, the researchers discovered decreased expression of synaptic proteins PSD95 and synaptophysin in sleep-deprived mice, reversible with dexmedetomidine. Golgi staining corroborated this reversal.
Microglia-mediated synaptic pruning
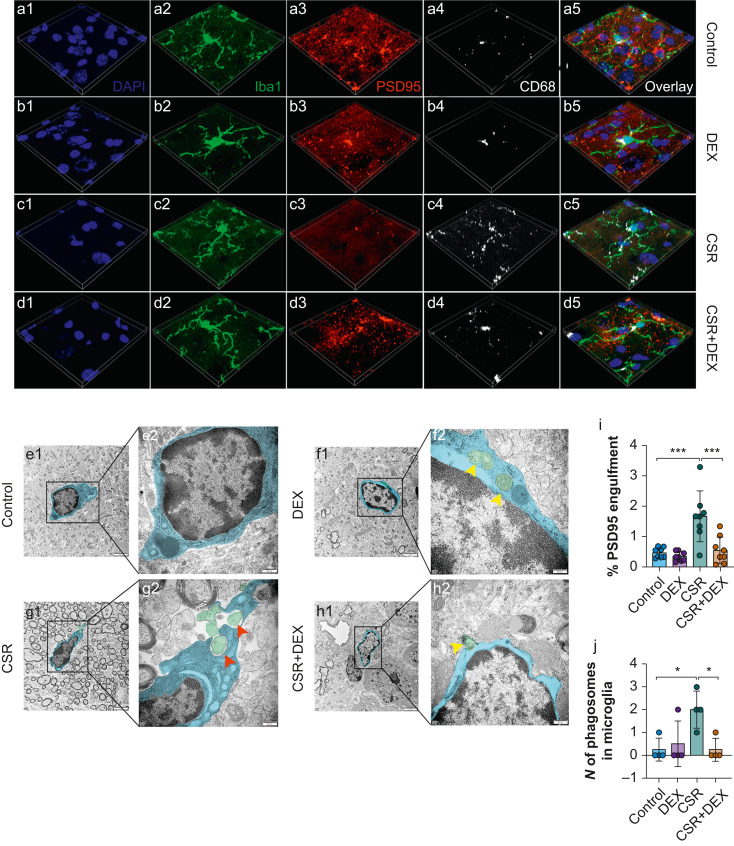
The researchers sought to determine if microglia-mediated synaptic pruning could underpin the observed synaptic changes. They found sleep deprivation activated microglia and substantially upregulated expression of complement proteins C1q, C3 and C3aR. Meanwhile dexmedetomidine could suppress microglial activation and C3, C3aR levels. This pointed to complement-directed microglial synaptic elimination as a potential participant in the underlying mechanisms.
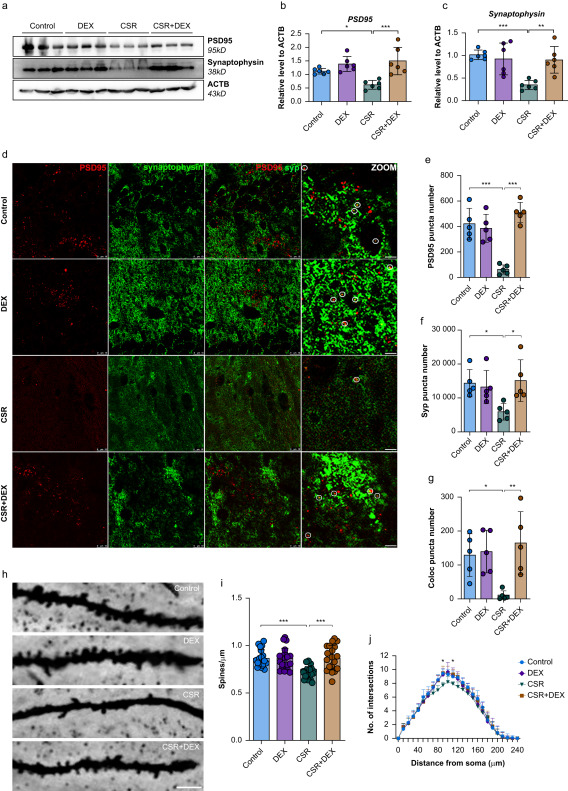
Further probing microglial phagocytic function uncovered increased engulfment of synapses and exacerbated synaptic loss in sleep-deprived mice. In contrast, dexmedetomidine inhibited microglial synapse phagocytosis. This lent additional confirmation of complement-mediated microglial synaptic elimination as a key basis for cognitive decline after sleep loss, with dexmedetomidine remediating cognition by arresting synaptic removal. Even though dexmedetomidine impedes complement signaling, a question lingered - would its effects persist after blocking C3-C3aR? By administering the C3aR inhibitor SB290157, the researchers found obstructing C3-C3aR similarly reversed cognitive deficits and synapse loss in sleep-restricted mice, on par with dexmedetomidine alone.
Finally, the team investigated whether dexmedetomidine restrains complement-driven synaptic loss by activating α2 adrenergic receptors in the brain. In the hippocampus, α2A receptors predominated exclusively on astrocytes. Via intracranial injection of the α2A receptor inhibitor BRL-44408, the researchers found dexmedetomidine's cognitive enhancement, reduced complement proteins C3 and C3aR, and diminished microglial phagocytosis were all blocked by BRL-44408. This signified that dexmedetomidine mediates its rescuing effects on cognitive deficits through astrocytic α2A receptors, while inhibiting the C3-C3aR pathway.
This study first revealed dexmedetomidine activates astrocytic α2A receptors to suppress their release of C3, which acts on microglial C3aR to inhibit synapse phagocytosis, reducing synaptic loss after sleep deprivation and thereby rescuing cognitive dysfunction. These findings provide novel insights into dexmedetomidine’s improvement of cognitive impairments. Together, this indicates dexmedetomidine may exert a dual benefit of strengthening sleep quality and recovering cognitive function, potentially becoming a preferred insomnia therapy and delivering welcome news for sleepless patients.
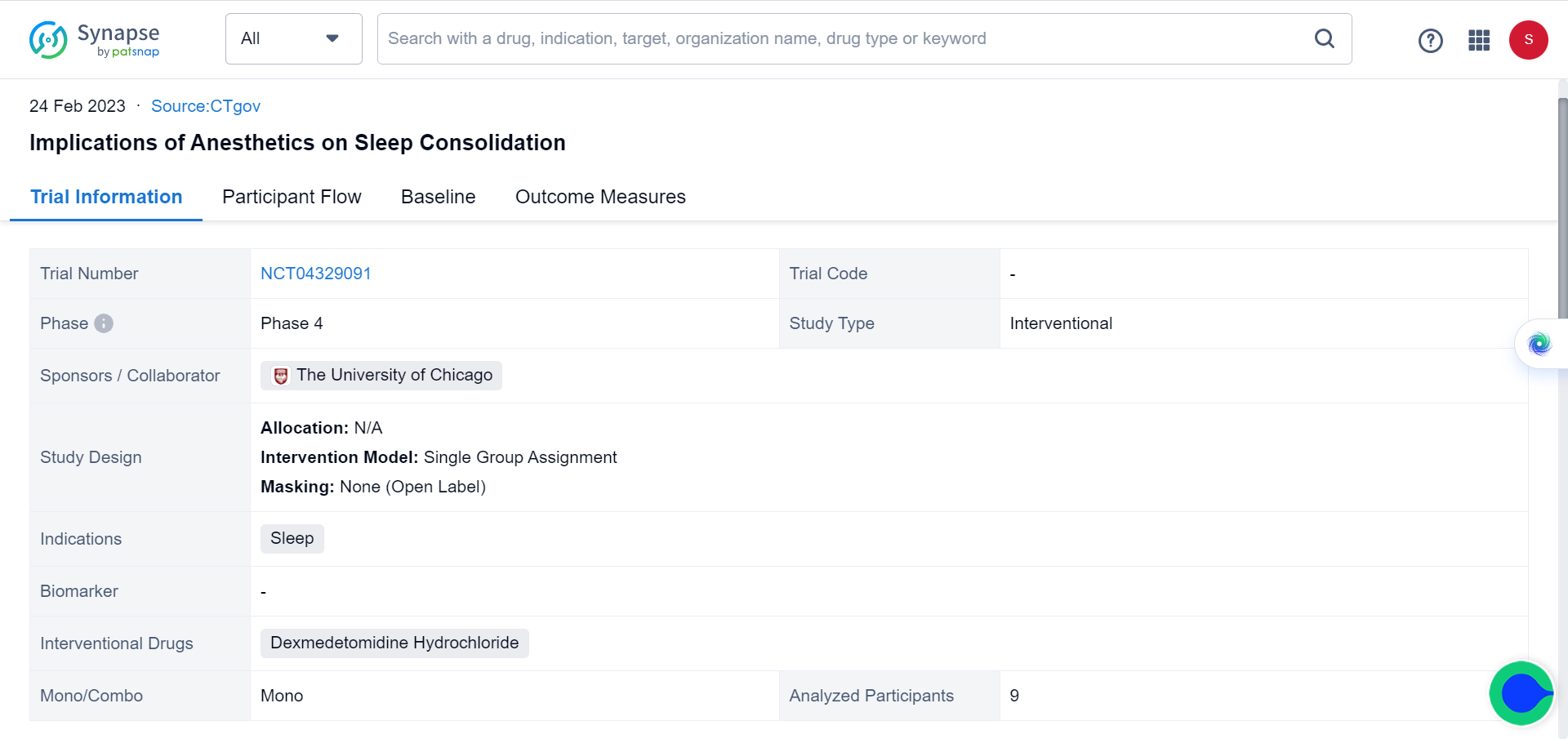
Meanwhile, the effects of dexmedetomidine on sleep and cognition have attracted other researchers. The Synapse database documents a team from the University of Chicago currently testing the implications of dexmedetomidine on sleep consolidation, with trials well into phase 4. As various measurable brain activities are associated with memory consolidation during sleep, their project aims to elucidate the effect of dexmedetomidine on memory consolidation during sleep.
While dexmedetomidine's ability to rescue cognition from the clutches of sleep deprivation offers exciting therapeutic potential, further research is still needed to fully elucidate its mechanisms and translate these findings to clinical applications. As researchers around the world advance our understanding of this multifaceted drug, dexmedetomidine promises to occupy an important place in the pharmacopeia, delivering restful repose to insomnia sufferers while also preserving their cognitive faculties. The coming years will reveal whether this sedative-stimulant can fulfill its potential and become a preferred therapy for sleepless patients worldwide.
Reference
Zhai Q, Zhang Y, Ye M, et al. Reducing complement activation during sleep deprivation yields cognitive improvement by dexmedetomidine [published online ahead of print, 2023 Jul 28]. Br J Anaesth. 2023; S0007-0912(23)00281-7.