Incendia Therapeutics Initiates Phase 1c Trial for Innovative DDR1 Inhibitor, PRTH-101
Incendia Therapeutics, a company specializing in precision oncology and focused on discovering and developing new therapies that alter the tumor microenvironment (TME), evealed the enrollment of the initial patient in their Phase 1c trial of PRTH-101 for individuals with advanced or metastatic solid tumors.
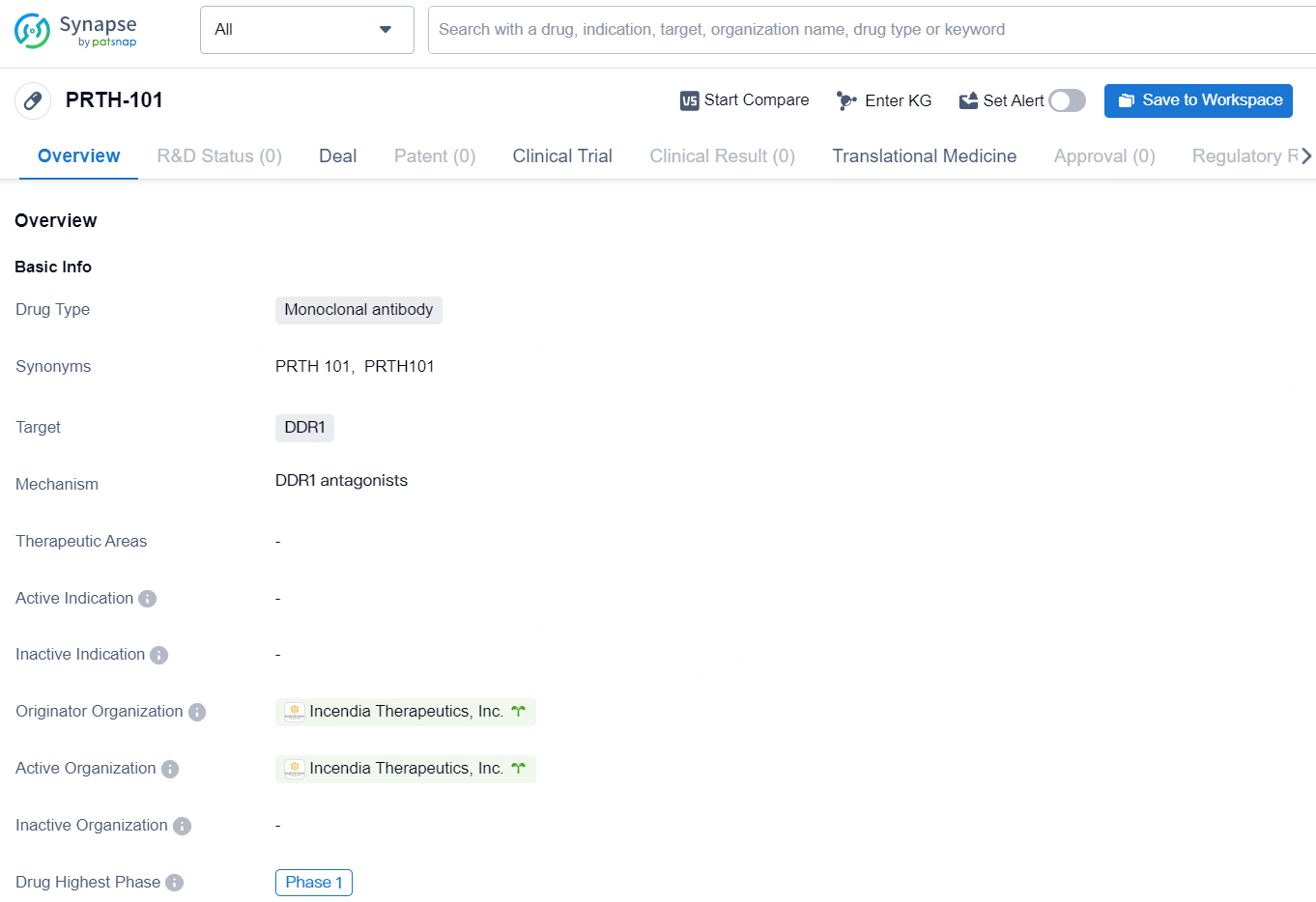
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“In our Phase 1a and 1b trials, we achieved the selection of an optimal dose and identified potential biomarkers to advance,” stated Irena Webster, SVP Development and Operations of Incendia. “The insights from this study will guide the Phase 2/3 program design, for which planning and global feasibility assessments are currently in progress.”
Dr. Joseph Paul Eder, CMO, commented, “Through the efforts of Incendia's exceptional team of researchers and clinical sites, we have established a recommended Phase 2 dose of PRTH 101. PRTH 101 has exhibited a consistent safety profile both as a standalone treatment and when used in combination with pembrolizumab. Our focus will now be on tumor types that have shown evidence of clinical activity and patient benefit.”
The Phase 1c trial will assess the safety, tolerability, and anti-tumor efficacy of PRTH-101 as both a single agent and in combination with KEYTRUDA® (pembrolizumab).
PRTH-101 is a therapeutic antibody that targets DDR1, a collagen-binding protein and kinase found on epithelial cells. Tumor cells are thought to exploit DDR1-collagen binding to form a protective barrier around the tumor. By allosterically inhibiting DDR1, PRTH-101 disrupts tumor-associated collagen alignment, facilitating immune cell infiltration into the tumor core. In preclinical studies, PRTH-101 has shown anti-tumor activity as a monotherapy and significantly enhanced the efficacy of checkpoint inhibitors. Tumor types with high levels of DDR1-associated collagen barriers include thymic, colorectal, pancreatic, ovarian, glioma, and non-small cell lung cancer. Currently, no approved therapies specifically target DDR1.
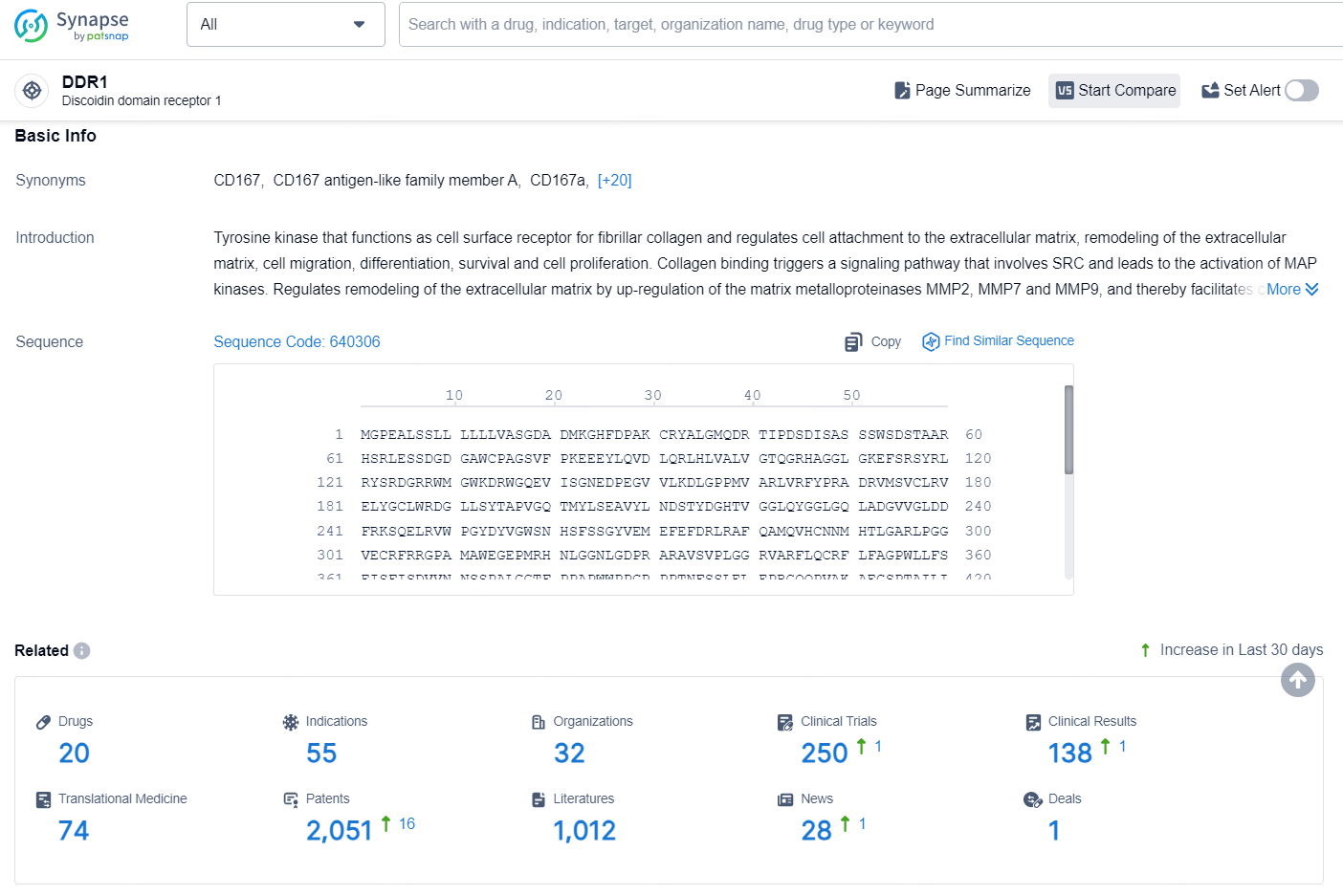
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 20 investigational drugs for the DDR1 target, including 55 indications, 32 R&D institutions involved, with related clinical trials reaching 250, and as many as 2051 patents.
PRTH-101 is a monoclonal antibody drug designed to target DDR1, a protein associated with various diseases including cancer and fibrosis. The drug is being developed by Incendia Therapeutics, Inc., a pharmaceutical organization specializing in biomedicine. As of the latest information available, PRTH-101 has reached the highest phase of clinical development globally, which is Phase 1.