Monoclonal antibodies targeting PACAP peptide for the treatment of migraine
On September 4th, an article titled "A Monoclonal Antibody to PACAP for Migraine Prevention" was published in the latest issue of The New England Journal of Medicine. The article reported on Phase 2 clinical trial data for the use of the monoclonal antibody Lu AG09222ALD-1910 targeting pituitary adenylate cyclase-activating polypeptide (PACAP) in the treatment of migraine.
It is reported that Lu AG09222(ALD-1910), a monoclonal antibody against PACAP, was originally developed by Lundbeck Seattle BioPharmaceuticals (LSBP). It functions by inhibiting the signaling pathways mediated by multiple G protein-coupled receptors (GPCRs) downstream of PACAP.
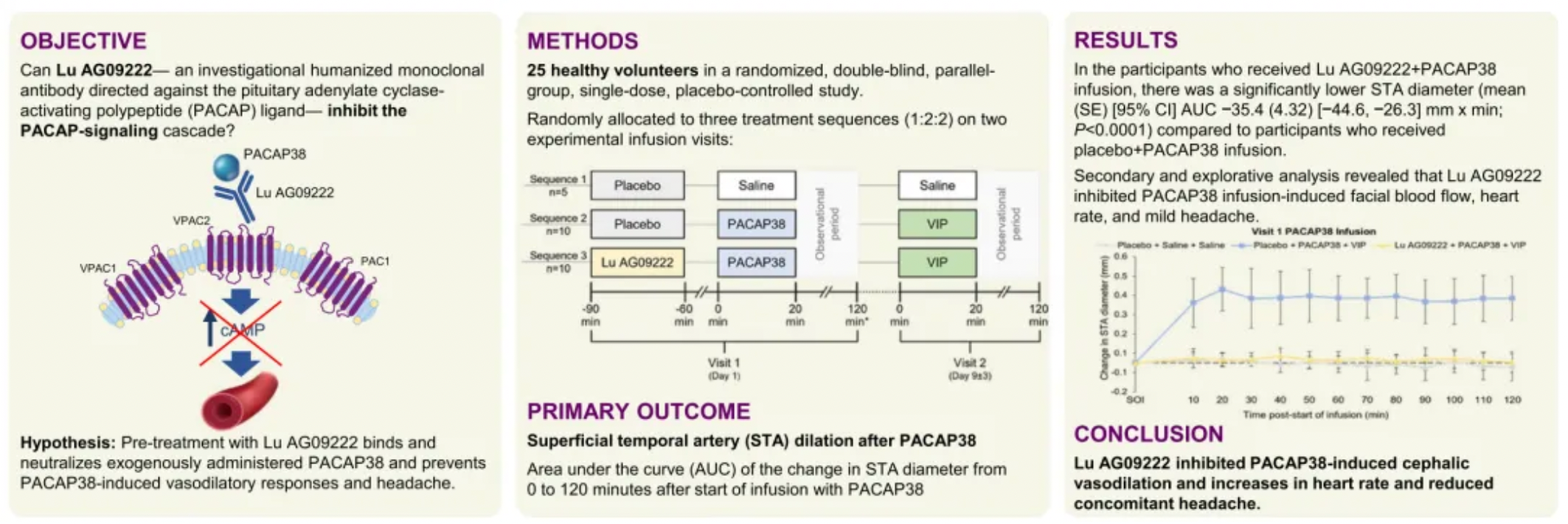
In 2019, the Danish pharmaceutical company H. Lundbeck acquired LSBP and, consequently, took ownership of one of their products. Lundbeck continued to advance the clinical development of this product for migraine treatment. According to updated Phase 1 clinical trial data from 2023, healthy participants who received injections of Lu AG09222 + PACAP38 showed that Lu AG09222 inhibited PACAP38-induced dilation of head blood vessels and an increase in heart rate, and also reduced the associated headaches. These findings suggest that Lu AG09222 could be a potential treatment method for migraines and other PACAP-mediated diseases.
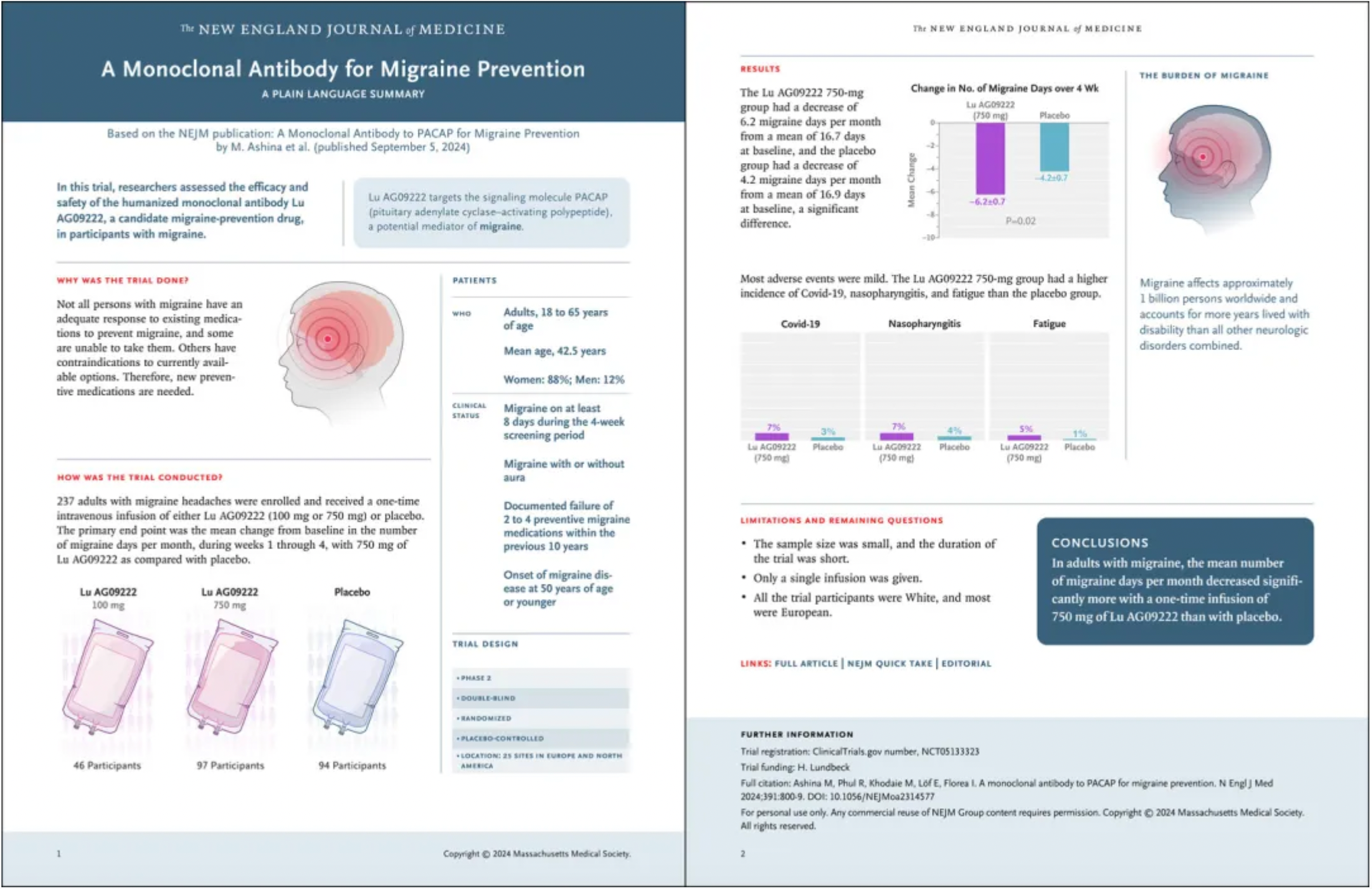
In this Phase 2, double-blind, randomized, placebo-controlled trial reported by the New England Journal of Medicine (NEJM), subjects were adults aged 18 to 65 years old with migraines, who had not benefited from 2 to 4 previous preventive treatments. Participants were randomly assigned in a 2:1:2 ratio to receive a single dose infusion of 750 mg Lu AG09222, 100 mg Lu AG09222, or a placebo.
The results showed that a total of 237 participants enrolled, with 97 receiving 750 mg Lu AG09222, 46 receiving 100 mg Lu AG09222, and 94 receiving the placebo. The average number of migraine days per month at baseline across the entire population was 16.7 days. In the 750 mg Lu AG09222 group, the average change from week 1 to 4 was a reduction of 6.2 days, compared to a reduction of 4.2 days in the placebo group (difference of 2.0 days; 95% confidence interval, -3.8 to -0.3; P=0.02).
In terms of safety, during a 12-week observation period, the more common adverse events in the 750 mg Lu AG09222 group compared to the placebo group included COVID-19 (7% vs. 3%), nasopharyngitis (7% vs. 4%), and fatigue (5% vs. 1%).
According to reports, migraine is a common chronic cerebrovascular neurological disorder that significantly impacts both individuals and society. It affects approximately one billion people worldwide and is characterized by recurrent, highly disabling episodes accompanied by severe headaches, nausea, vomiting, extreme sensitivity to light and sound, and various other physical, mental, and psychological signs and symptoms.
Traditionally, the pathway involving CGRP (Calcitonin Gene-Related Peptide) and its receptors is considered one of the key pathways for migraine treatment, with several drugs already approved for marketing. In addition to this, targets like 5-HTR (5-Hydroxytryptamine Receptors) and COX (Cyclooxygenase) are also potential targets for clinical drug development.
The article mentions PACAP (Pituitary Adenylate Cyclase-Activating Polypeptide) as an emerging potential target for migraine treatment. Besides the monoclonal antibodies mentioned in the article currently in Phase II clinical trials, Eli Lilly's development of Vipalanebart is also in Phase II clinical study stage.
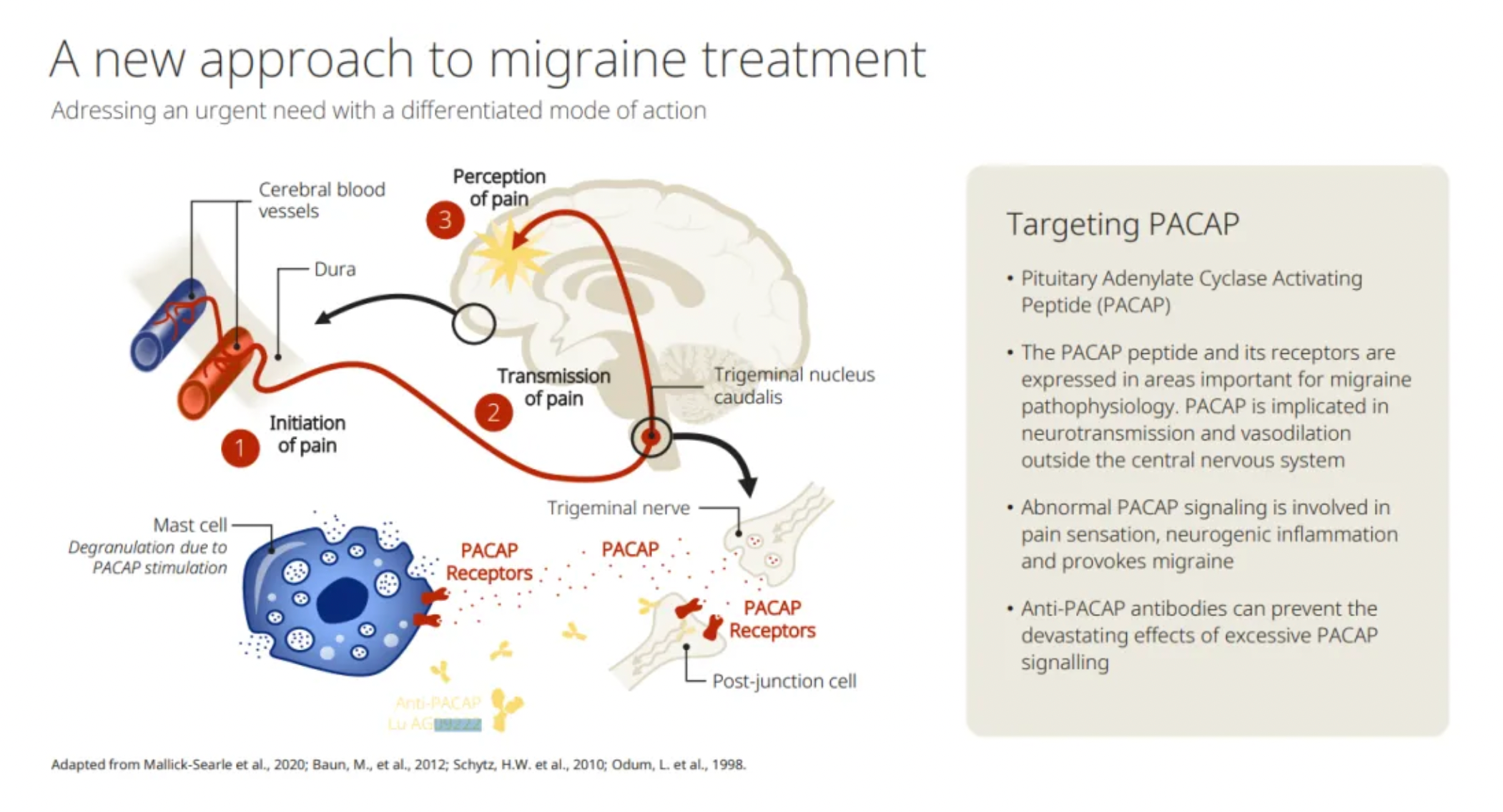
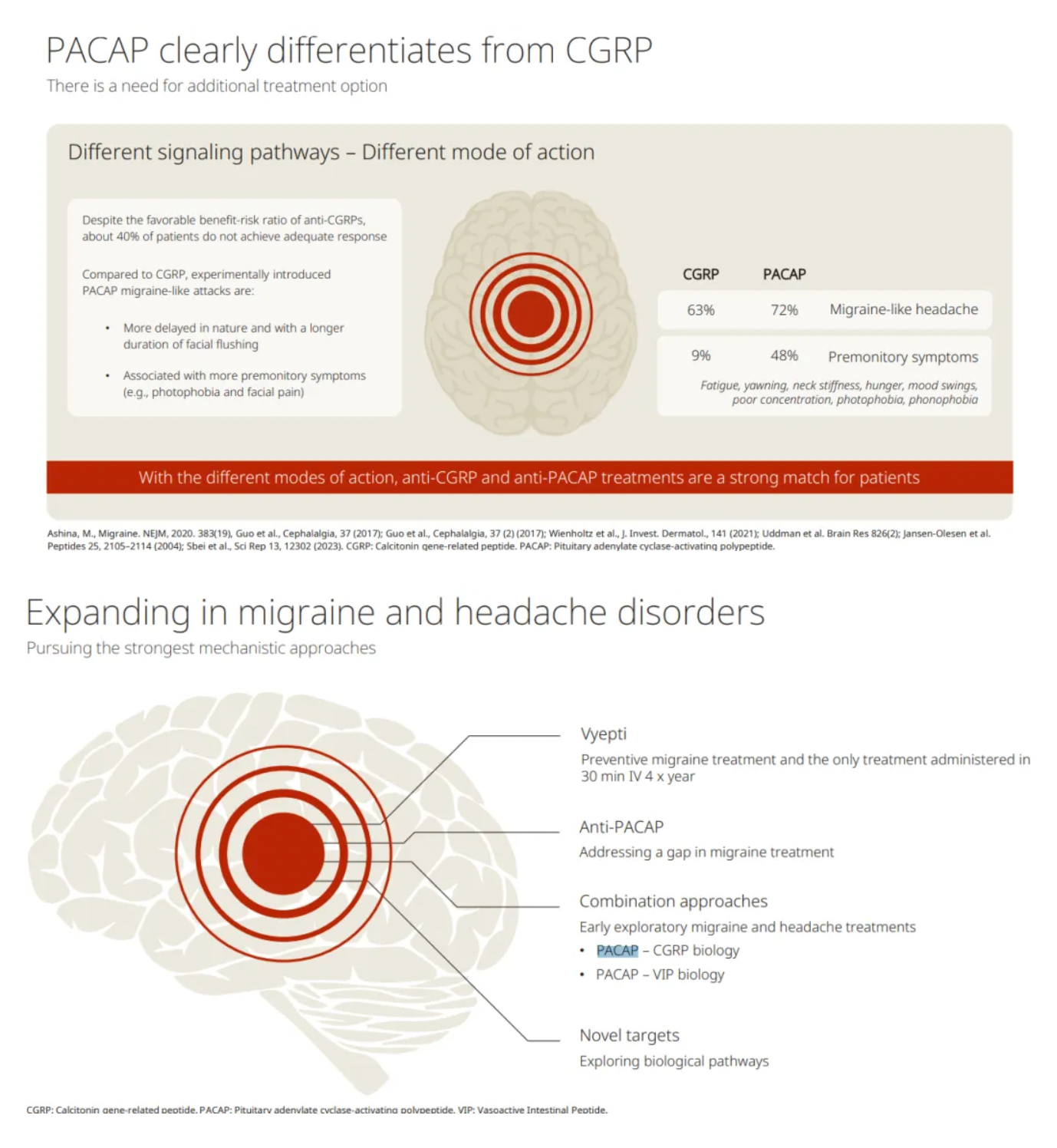
The differences in treating migraine with PACAP and CGRP are highlighted.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!