Viridian Therapeutics Announces Positive Phase 3 Results for Veligrotug in Thyroid Eye Disease
Viridian Therapeutics, Inc. (NASDAQ: VRDN), a biopharmaceutical company dedicated to the discovery and development of potentially best-in-class medications for severe and rare diseases, has reported encouraging topline results from the THRIVE phase 3 clinical trial of VRDN-001, now referred to as veligrotug. Veligrotug is an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody administered intravenously for the treatment of patients with active thyroid eye disease (TED). TED is an autoimmune disorder marked by inflammation, hypertrophy, and damage to the tissue surrounding and located behind the eyes.
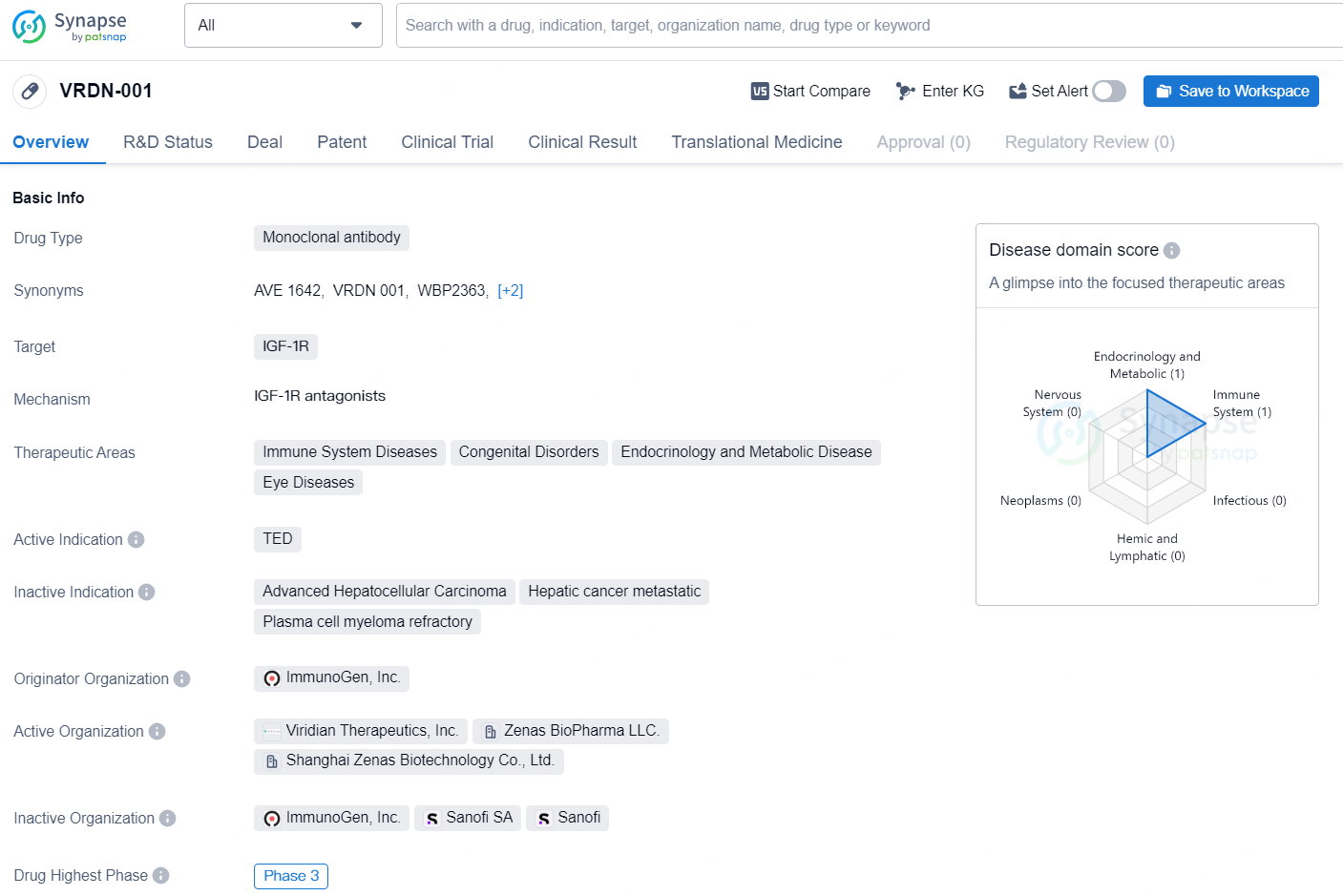
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are delighted with the remarkable THRIVE top-line results, having successfully met our stringent standards for both efficacy and safety. Notably, veligrotug demonstrated a swift onset of clinically significant responses across all measured endpoints, surpassing our safety expectations. Veligrotug's unique and condensed five-infusion dosing schedule presents a potentially more convenient treatment alternative with reduced intravenous burden for patients, compared to existing standards," remarked Steve Mahoney, President and CEO of Viridian.
"Our enthusiasm for the results extends beyond veligrotug to our other clinical asset in the TED franchise, VRDN-003, which is administered subcutaneously. Last month, we launched two phase 3 clinical trials for VRDN-003. We believe that both veligrotug and VRDN-003 could become the preferred therapies for TED patients. The robust results from THRIVE also bolster our confidence in VRDN-003, given it shares the same binding domain as veligrotug, and could be a leading option as an infrequent subcutaneous injection. This has the potential to revolutionize treatment for TED patients and widen market scope. Our team has performed exceptionally in the THRIVE studies, surpassing enrollment targets globally, including in the U.S., which assures us that recruitment for the VRDN-003 trials, REVEAL-1 and REVEAL-2, will proceed quickly. Additionally, we are enthusiastic about our developing FcRn inhibitor portfolio and plan to share more details later this year."
"These phase 3 clinical data underscore the significant clinical activity of veligrotug. The data highlighted significant improvements in proptosis, clinical activity score, and diplopia in TED patients after only five infusions. This aligns with the clinical benefits observed from IGF-1R antagonism in TED. Veligrotug also exhibited a fast onset of action, which is likely to be beneficial for patients," stated Michael Yen, M.D., a THRIVE investigator and Professor of Oculoplastic Surgery and Ophthalmology at Baylor College of Medicine. "In this large phase 3 trial, veligrotug demonstrated a favorable safety profile, even with close monitoring for possible hearing impairment. As a THRIVE investigator, I am encouraged by these results and look forward to the REVEAL trials for VRDN-003, which could offer additional treatment options for individuals with TED."
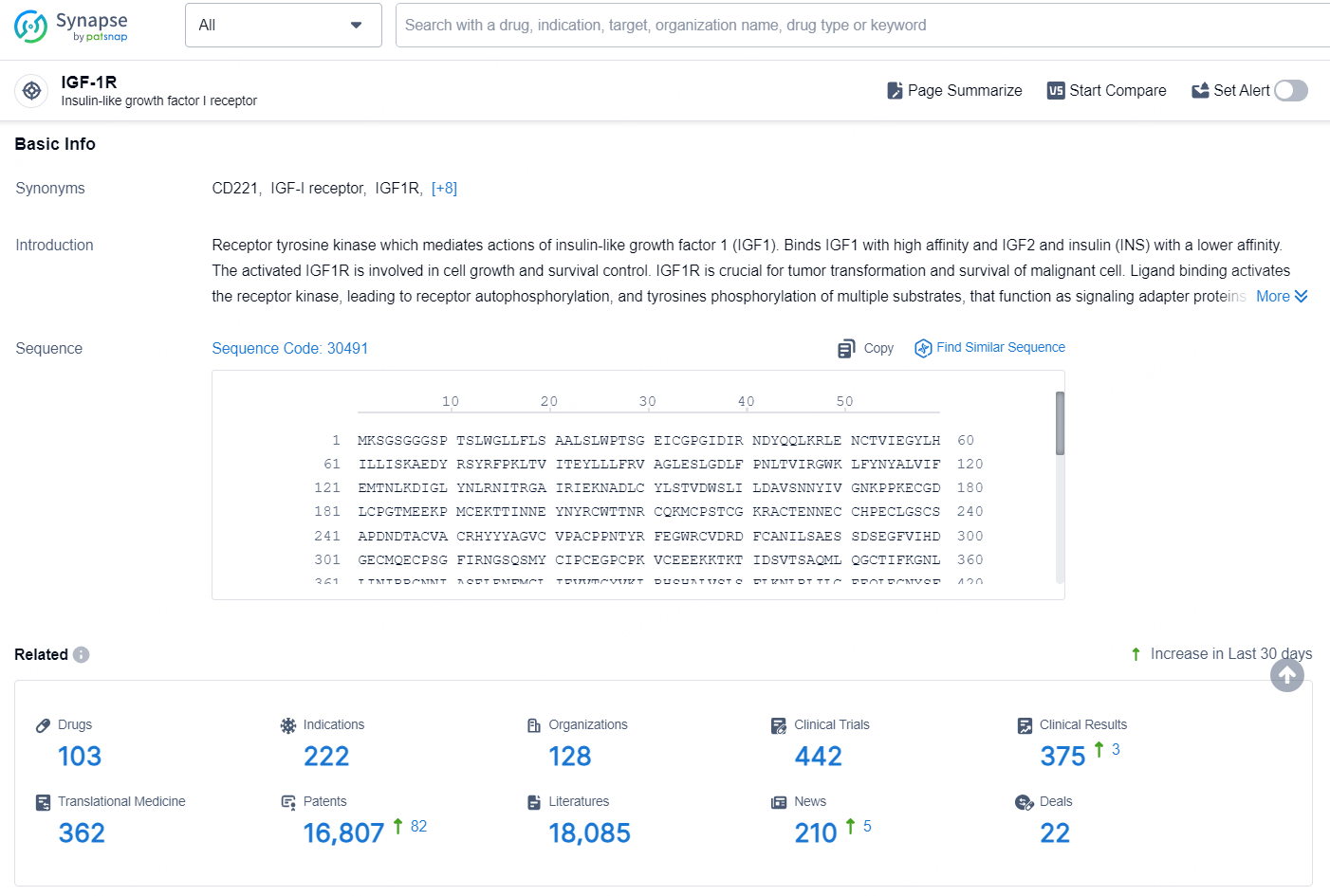
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 11, 2024, there are 103 investigational drugs for the IGF-1R targets, including 222 indications, 128 R&D institutions involved, with related clinical trials reaching 442, and as many as 16807 patents.
VRDN-001 is a monoclonal antibody drug developed by ImmunoGen, Inc. It targets IGF-1R and is intended for use in the treatment of immune system diseases, congenital disorders, endocrinology and metabolic disease, and eye diseases. Its active indication is TED (Thyroid Eye Disease). As of the latest available information, the drug has reached Phase 3 in its development process globally, indicating that it has undergone extensive testing in human subjects to evaluate its safety and efficacy. In China, the highest phase that VRDN-001 has reached is Phase 1/2, suggesting that the drug is in the early stages of testing and development within the Chinese market.