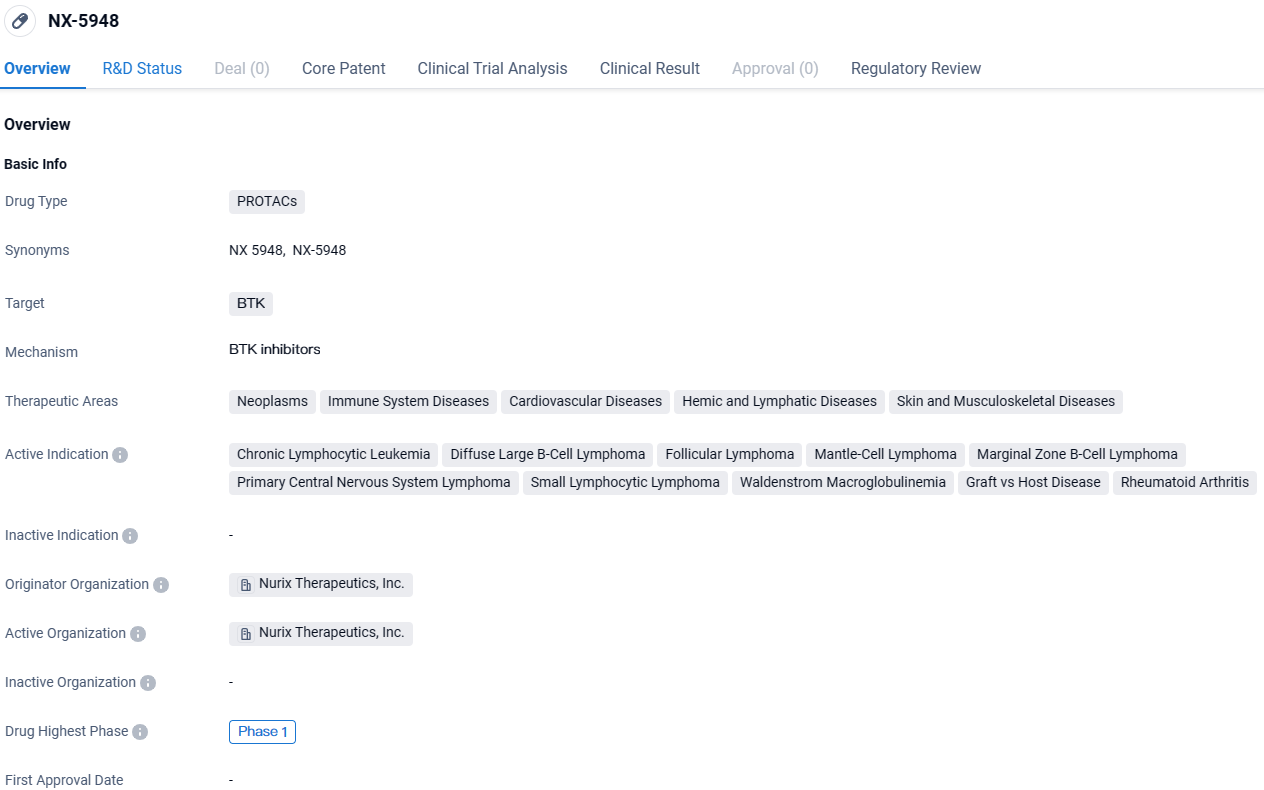
Initial Clinical Evidence of Brain Activity for Nurix's Oral BTK Inhibitor NX-5948 in B Cell Cancer Treatment
Nurix Therapeutics, Inc., an enterprise focused on clinical advancement in the biopharmaceutical field, is actively working on crafting drugs that modulate specific proteins. These drugs are intended to provide treatment for individuals suffering from oncological conditions and inflammation-related illnesses. The company has recently publicized initial clinical results pertaining to the effectiveness of NX-5948 in cerebral responses. NX-5948 is an orally administered compound that precisely targets and causes the degradation of Bruton's tyrosine kinase (BTK).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The symposium showcased analytical reviews of two individual cases: one involving a patient with Chronic Lymphocytic Leukemia (CLL) with complications affecting the Central Nervous System (CNS), and another dealing with a Primary Central Nervous System Lymphoma (PCNSL) sufferer. Both subjects experienced substantial therapeutic benefits. Additionally, the symposium highlighted the detection of active pharmaceutical concentrations in the CNS of several participants with CNS tumors, all part of the active Phase 1 study. These findings were introduced by Dr. Gwenn M. Hansen, the lead scientific executive at Nurix. This was during her talk at the Major Symposium titled "Molecular Glues, PROTACs, and Next-Generation Degraders: Discoveries and Early Preclinical Developments" during the American Association for Cancer Research (AACR) Annual Meeting 2024, from April 5th to 10th in San Diego, California.
Dr. Hansen remarked, "This represents the premier instance of demonstrating a targeted protein degrader's efficacy within the CNS, paving new possibilities for treatment regimens for leukemias and lymphomas affecting the CNS." She advocated for the drug NX-5948, given its ability to penetrate the brain and its observed efficacy and safety in treating difficult CNS-related B-cell lymphomas and chronic lymphocytic leukemia. She also proposed NX-5948 as a potential treatment for CNS-related immune disorders, such as multiple sclerosis.
Dr. Hansen exhibited fresh insights from the dose amplification segment of Nurix's Phase 1a/1b clinical assessment, which involved the daily oral administration of the Bruton's Tyrosine Kinase (BTK) degrader, NX-5948, for patients with resistant or recurring B-cell malignancies. It was shown that NX-5948 was present in every cerebrospinal fluid sample that was tested. Two patients’ case analyses were also discussed in the presentation.
Dr. Arthur T. Sands, CEO and President of Nurix, stated, "The preliminary clinical results observed with NX-5948 in individuals with severe CNS diseases justify further investigation of the drug, both alone and combined with other treatments for CNS lymphoma and leukemia." He emphasized an impressive, enduring response in a CLL patient with CNS challenges using NX-5948 monotherapy. A considerable, swift remission in a PCNSL patient with aggressive Non-Hodgkin's Lymphoma (NHL) histology was highlighted, showcasing the drug’s promise for efficacy within the CNS that may be enhanced through combined treatment strategies to extend the response duration."
NX-5948 is an experimental, orally bioavailable, CNS-penetrating small molecule designed to degrade BTK. Currently, the compound is undergoing a Phase 1 clinical trial in patients with refractory or recurrent B cell malignancies. Earlier disclosures by Nurix have claimed that NX-5948 shows exceptional potency against various tumor cell lines that resist standard BTK inhibitor treatments—a critical factor for the treatment of CLL/Small Lymphocytic Lymphoma (SLL) cohorts with extensive prior treatments.
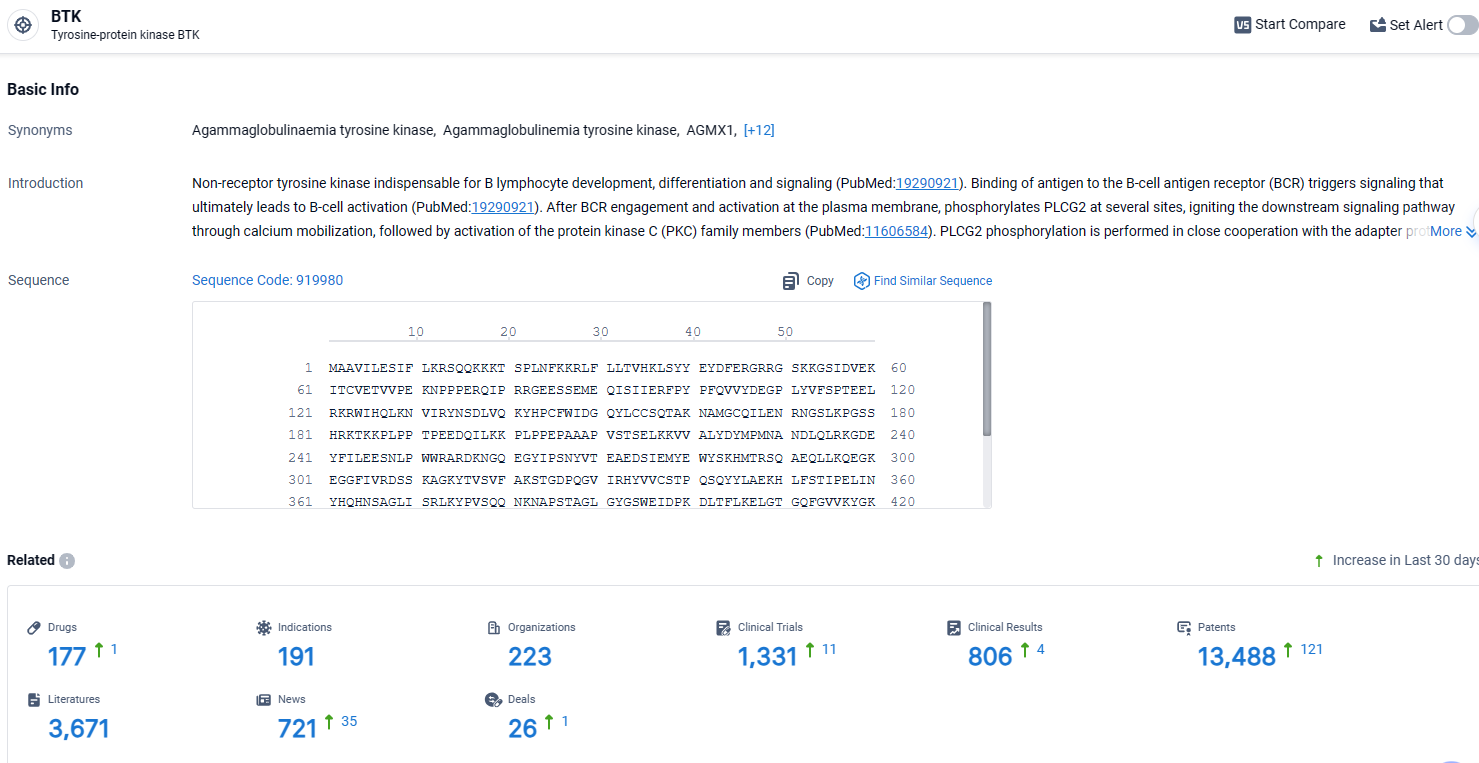
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 11, 2024, there are 177 investigational drugs for the BTK target, including 191 indications, 223 R&D institutions involved, with related clinical trials reaching 1331, and as many as 13488 patents.
NX-5948 targets BTK and has potential applications in various therapeutic areas, including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The drug is currently in Phase 1 of clinical trials and has been granted Fast Track designation, highlighting its potential as a promising treatment option for a range of diseases.