Initial Dosing in ReCode's Phase 1 Trial of Inhaled mRNA Therapy, RCT2100, for Cystic Fibrosis
ReCode Therapeutics has declared the commencement of a Phase 1 clinical trial for their novel treatment, RCT2100, with the initial group of healthy volunteers already receiving their doses. This groundbreaking therapy is an inhaled form of mRNA designed to target the treatment of cystic fibrosis, particularly attending to the subset of 10-13% of patients harboring nonsense mutations. These patients typically exhibit no response or adverse reactions to the existing approved CFTR modulator therapies.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Launching the clinical trial for RCT2100, our second drug candidate crafted using the revolutionary Selective Organ Targeting (SORT) lipid nanoparticle platform, marks a significant advancement for the Cystic Fibrosis (CF) population," announced Dr. David Lockhart, the distinguished Chief Scientific Officer and President at ReCode Therapeutics.
Dr. Lockhart expressed, "Despite strides forward in the treatment for many CF patients, individuals unresponsive to current modulators often feel overlooked, enduring severe physical and psychological burdens due to the condition. RCT2100 is engineered to address the fundamental cause of CF, promising to extend potential benefits to a broader spectrum of patients, particularly those with uncommon mutations."
Cystic Fibrosis arises from genetic anomalies in the CFTR gene, affecting roughly 130,000 individuals globally. While there have been improvements in therapies targeting CFTR modulation, no treatments to date can rectify Class I mutations which yield no functional CFTR protein amenable to modulation. Leveraging the proprietary SORT LNP platform from ReCode, inhaled RCT2100 aims to deliver CFTR mRNA directly to cells, directing them to synthesize a functional CFTR protein.
The underway Phase 1 study, a double-blind and placebo-controlled trial, aims to appraise the safety and endurance of different strengths of RCT2100 when administered as a single inhaled dose through a nebulizer. Conducted in New Zealand, the trial by ReCode is set to include roughly 32 healthy volunteers, who will be randomly assigned to receive either a dose of RCT2100 or a placebo.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
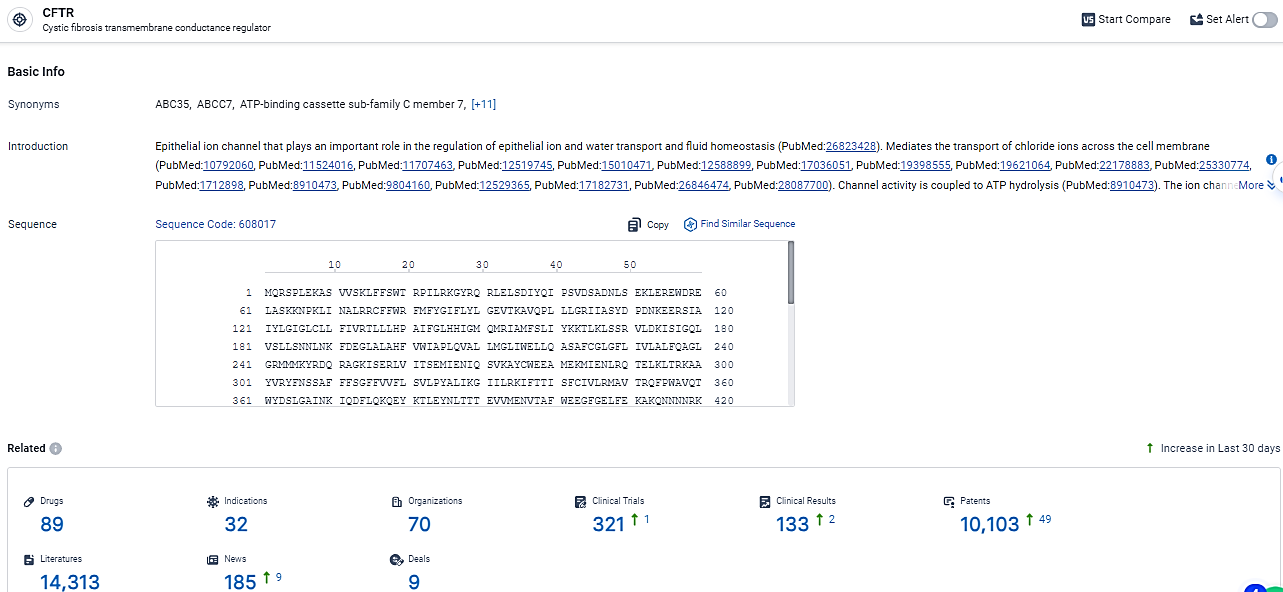 According to the data provided by the Synapse Database, As of February 28, 2024, there are 89 investigational drugs for the CFTR target, including 32 indications, 70 R&D institutions involved, with related clinical trials reaching 321, and as many as 10103 patents.
According to the data provided by the Synapse Database, As of February 28, 2024, there are 89 investigational drugs for the CFTR target, including 32 indications, 70 R&D institutions involved, with related clinical trials reaching 321, and as many as 10103 patents.
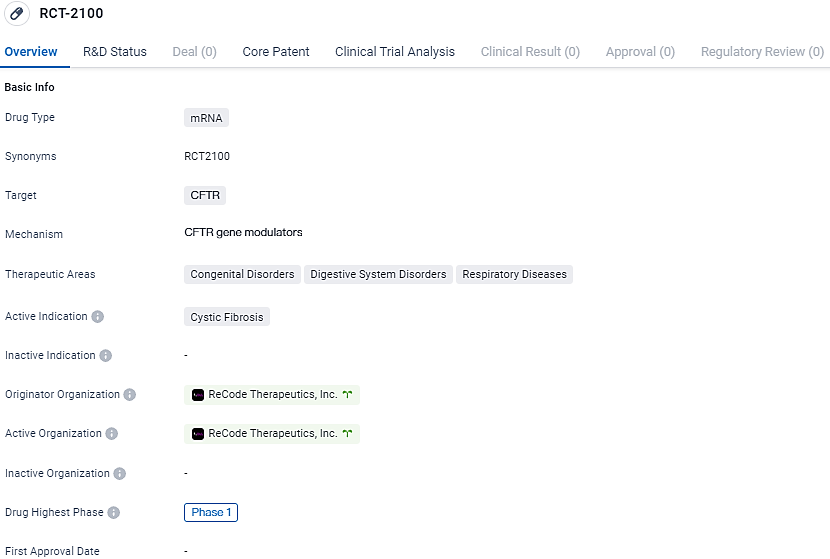
RCT-2100 target CFTR and treat Cystic Fibrosis. It is currently in Phase 1 of development and aims to address the therapeutic areas of Congenital Disorders, Digestive System Disorders, and Respiratory Diseases. Further research and clinical trials will be necessary to determine the drug's efficacy and potential for commercialization.