FDA Approves Biweekly TECVAYLI® for Refractory Multiple Myeloma
Johnson & Johnson has declared that the U.S. Food and Drug Administration granted authorization for an updated dosing regimen of TECVAYLI® (teclistamab-cqyv). Under the new protocol, the medication can be administered at a rate of 1.5 mg/kg biweekly to individuals with relapsed or refractory multiple myeloma who have secured and sustained at least a complete response for a duration no less than six months.
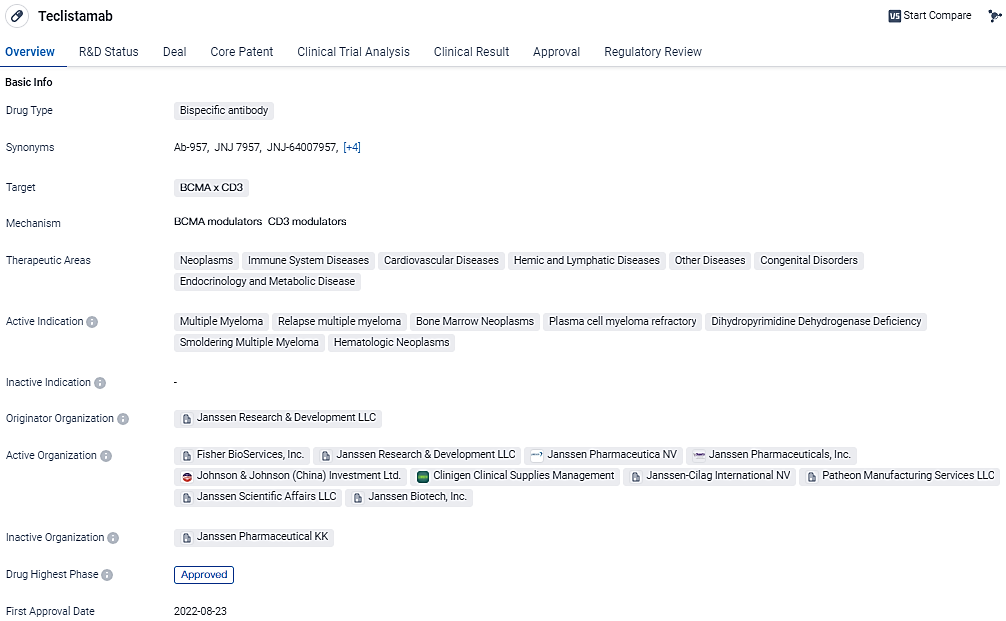
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
TECVAYLI® (teclistamab-cqyv), a bispecific antibody, was designed to target both the BCMA present on multiple myeloma cells and CD3 found on T-cells. This unique mechanism is intended to trigger an immunological attack on the cancer cells. Authorized for public use in October 2022, TECVAYLI® is reserved for those cases of multiple myeloma that have relapsed or are resistant to treatment (RRMM) in adults, with the stipulation that patients must have previously completed a minimum of four distinct therapeutic regimens. These should comprise treatment with a class of compounds known as proteasome inhibitors, medicines with immunomodulatory properties, and therapies involving anti-CD38 monoclonal antibodies.
The U.S. FDA granted TECVAYLI® a preliminary green light based mainly on the observed rates of response in patients; this provisional approval will be subject to further assessment based on proofs of ongoing clinical benefits that emerge from subsequent studies. Since its market introduction, over 3,600 U.S. patients have started treatment with TECVAYLI®.
The clinical evidence leading to TECVAYLI®'s authorization emerged from data collected in the MajesTEC-1 trial, which encompassed both Phase 1 and Phase 2 exploratory studies. Initially, participants received a standard Phase 2 dosage, which was 1.5 mg/kg of TECVAYLI® administered via subcutaneous injection on a weekly basis. For individuals who demonstrated a durable Complete Response (CR) for at least half a year, study protocols permitted a reduction in the frequency of their injections to once every two weeks (1.5 mg/kg Q2W), continuing unless the disease advanced further or the side effects became intolerable.
In summary, TECVAYLI®, the pioneering bispecific antibody targeting BCMA and CD3, represents a significant therapeutic option for adults with heavily pre-treated RRMM, and its continued use hinges on confirming its benefits in clinical trials.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
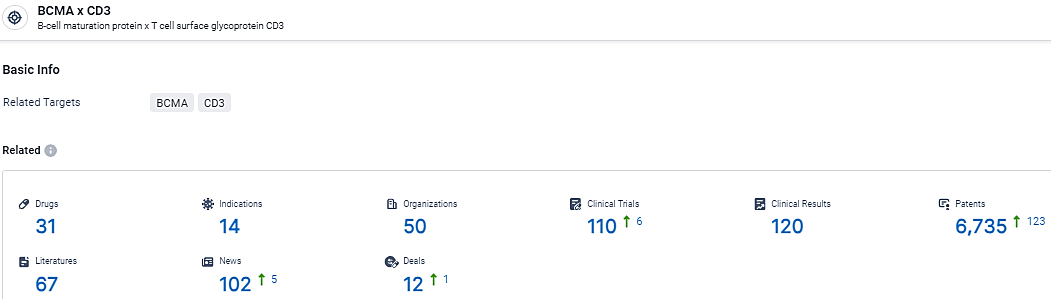 According to the data provided by the Synapse Database, As of February 26, 2024, there are 31 investigational drugs for the BCMA and CD3 target, including 14 indications, 50 R&D institutions involved, with related clinical trials reaching 110, and as many as 6735 patents.
According to the data provided by the Synapse Database, As of February 26, 2024, there are 31 investigational drugs for the BCMA and CD3 target, including 14 indications, 50 R&D institutions involved, with related clinical trials reaching 110, and as many as 6735 patents.
Teclistamab's approval in multiple countries and its ongoing regulatory process in China demonstrate its potential as a treatment option for patients with various hematologic malignancies. The drug's ability to target BCMA and CD3, coupled with its regulatory designations, positions it as a significant development in the field of biomedicine. Further studies and real-world evidence will be crucial in assessing the drug's long-term efficacy and safety profile.