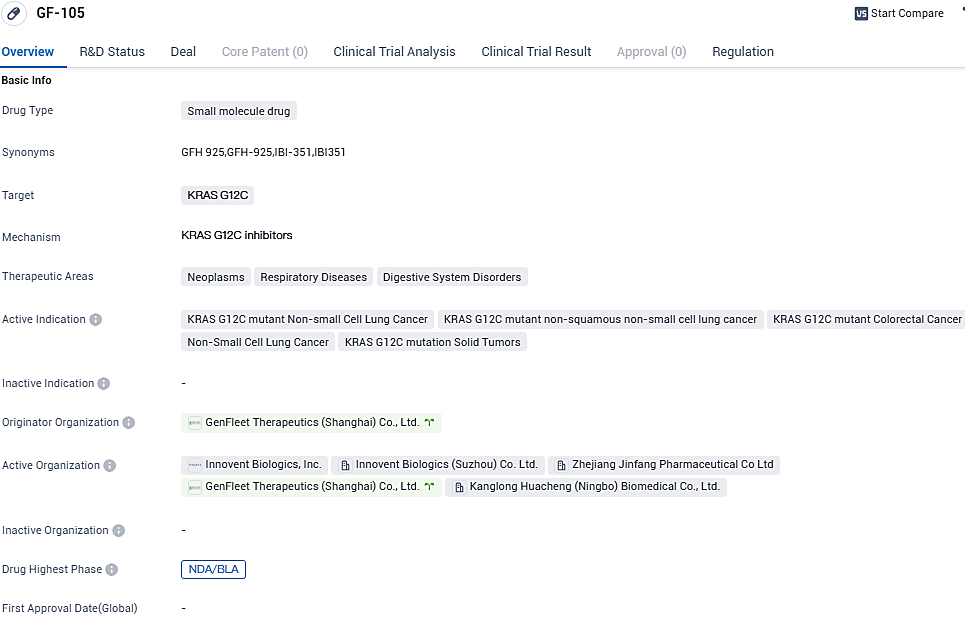
Innovent announces China's NMPA priority review of first domestic KRAS G12C inhibitor drug, IBI351
Innovent Biologics, Inc. has disclosed that the CDE of the NMPA in China has acknowledged the NDA for IBI351 (KRAS G12C inhibitor). It has additionally received Priority Review status for treating patients suffering from late-stage non-small cell lung cancer carrying KRAS G12C mutation, who have undergone a minimum of one systemic therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As China's pioneering NDA for a KRAS G12C inhibitor, it holds the potential to assist an expanded number of lung cancer patients possessing the KRAS G12C mutation with approval.
The acceptance of the NDA and the designation of Priority Review are premised on an individual, registrational Phase 2 clinical study's outcomes designed to determine the efficacy and safety of IBI351 monotherapy for advanced NSCLC patients with the KRAS G12C mutation who have not responded or were intolerant to standard treatment within China. These findings are set to be shared at the upcoming European Society for Medical Oncology Asia Congress in 2023.
While the FDA has authorized the use of KRAS G12C targeted drugs in foreign countries, no such drug holds approval within China. IBI351, serving as an innovative, irreversible covalent inhibitor of KRAS G12C mutation, exhibited a satisfactory safety profile along with potential efficacy in advanced NSCLC cases carrying KRAS G12C mutations solely treated with this drug.
Professor Yi-Long Wu from the Guangdong Lung Cancer Institute, Guangdong Provincial People's Hospital, commented: "The previously 'undruggable' target that was KRAS mutation has recently taken forefront in clinical development. We eagerly anticipate the NDA granting approval to this cutting-edge drug to aid more NSCLC patients genetic makeup includes the KRAS G12C mutation."
Dr. Hui Zhou, Innovent's Senior Vice President, remarked: "We warmly welcome the NDA acceptance of IBI351 as it can potentially rise to be the premier approved KRAS G12C inhibitor within China, broadening the treatment avenues for NSCLC patients. Additionally, we are pushing forward with the clinical development of IBI351 as a standalone treatment and integrated part of a treatment plan for other solid tumors such as colorectal and lung cancer, aiming to provide more patient assistance."
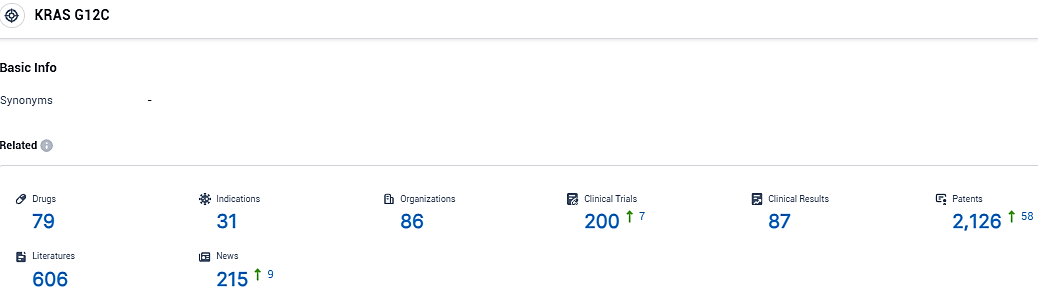
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 30, 2023, there are 79 investigational drugs for the KRAS G12C target, including 31 indications, 86 R&D institutions involved, with related clinical trials reaching 200, and as many as 2126 patents.
In January 2023, CDE of China's NMPA has granted Breakthrough Therapy Designation for IBI351 for the treatment of patients with advanced NSCLC harboring KRAS G12C mutation who have received at least one systemic therapy. In November 2023, the CDE of NMPA accepted and granted Priority Review designation to the NDA for IBI351 for the treatment of advanced NSCLC patients harboring KRAS G12C mutation who have received at least one systemic therapy.