Intense Competition and Innovative Developments in the Pan-KRAS Inhibitor Market
On September 23, 2024, Eli Lilly registered a Phase I clinical trial for its pan-KRAS inhibitor LY4066434 on Clinicaltrials.gov. Meanwhile, Jacobio’s independently developed pan-KRAS inhibitor JAB-23E73 received IND approval for clinical trials in China on September 26, 2024, following an earlier IND approval in the United States. Additionally, on September 30, 2024, BeiGene’s clinical trial application for its pan-KRAS inhibitor BGB-53038 capsules was also accepted by the NMPA. The involvement of three companies in pan-KRAS inhibitor clinical trials within just a few days demonstrates the intense competition in the development of pan-KRAS inhibitors.
The KRAS target is renowned in the field of cancer. In all tumors, approximately 30% are driven by RAS mutations, of which KRAS mutations account for up to 85%, earning it the title "King of Targets." KRAS, once considered one of the most challenging “undruggable” targets, finally lost that label with the 2021 approval of Amgen’s KRAS G12C inhibitor Lumakras. This breakthrough has since sparked widespread interest and enthusiasm among numerous companies for the development of KRAS-targeted therapies, making it a coveted area of drug development.
When discussing pan-KRAS inhibitors, it’s essential to mention RMC-6236, developed by Revolution Medicines. RMC-6236 is a tri-complex inhibitor targeting multiple RAS mutations and wild-type RAS. It binds to the intracellular chaperone protein cyclophilin A to create an inhibitory binary complex, which then forms a ternary complex with active GTP-bound RAS, disrupting interactions with effectors to inhibit RAS signaling. In July this year, Revolution Medicines reported positive results from a Phase 1b clinical trial of RMC-6236: as a monotherapy for second-line treatment of KRAS G12X mutant PDAC (pancreatic ductal adenocarcinoma), it achieved a median progression-free survival (PFS) of 8.1 months and a disease control rate (DCR) of nearly 90%. This is noteworthy compared to the median benchmarks of 2.0-3.5 months for most drugs. The positive progress of RMC-6236 underscores the significant potential of KRAS inhibitors in drug development.
Due to their importance in cancer treatment, pan-KRAS inhibitors have become a popular focus in pharmaceutical R&D, potentially leading to overlapping patents and competition among different companies. Next, we will take RMC-6236 as a starting point to explore patent overlaps among companies in the KRAS space using Patsnap Chemical.
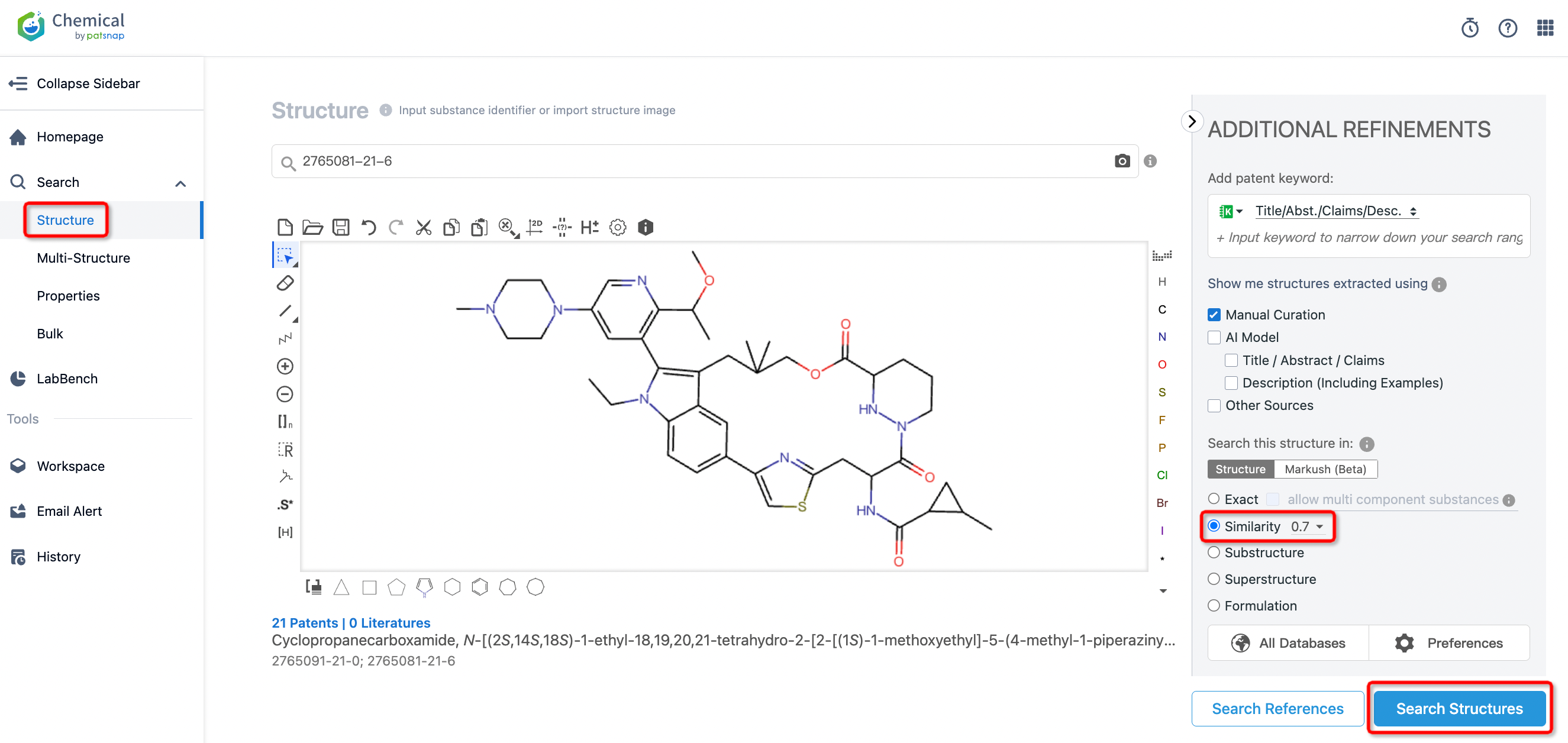
First, we log into Patsnap Chemical. By selecting structural search, we enter RMC-6236’s commonly used identifiers (such as CAS number, generic name, molecular formula, SMILES file, etc.). Alternatively, we can directly edit the structure in the editor or upload a structural file (MOL format) from ChemDraw. Here, we use a similarity search (setting the Tanimoto coefficient to 0.7) to identify patent applications structurally similar to RMC-6236.
After initiating the compound search, we find that the compound’s first publication date is March 24, 2022, with 33 related patents of similar structure.
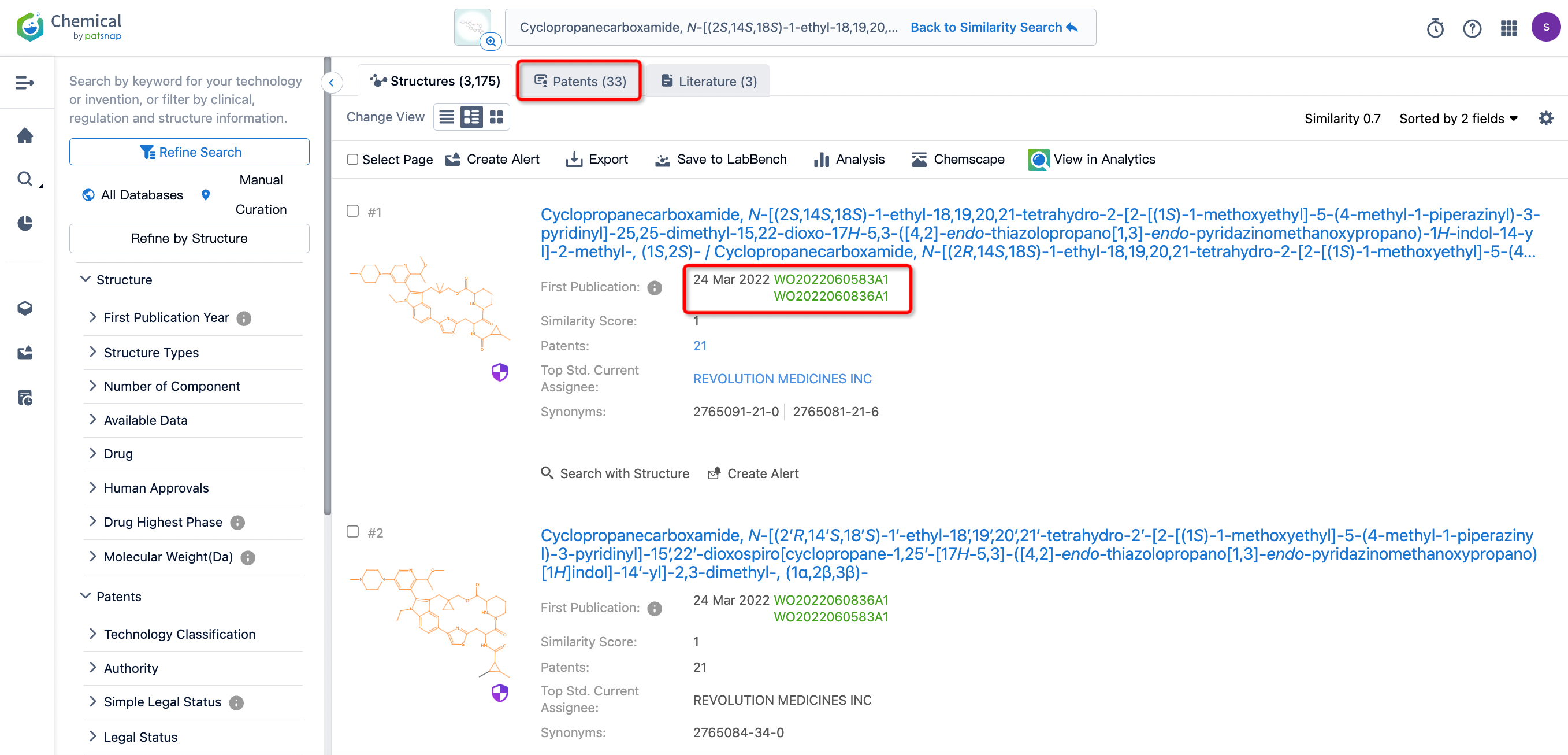
To view related patents conveniently, we can click on “View in Analytics” to link to the Patsnap database.
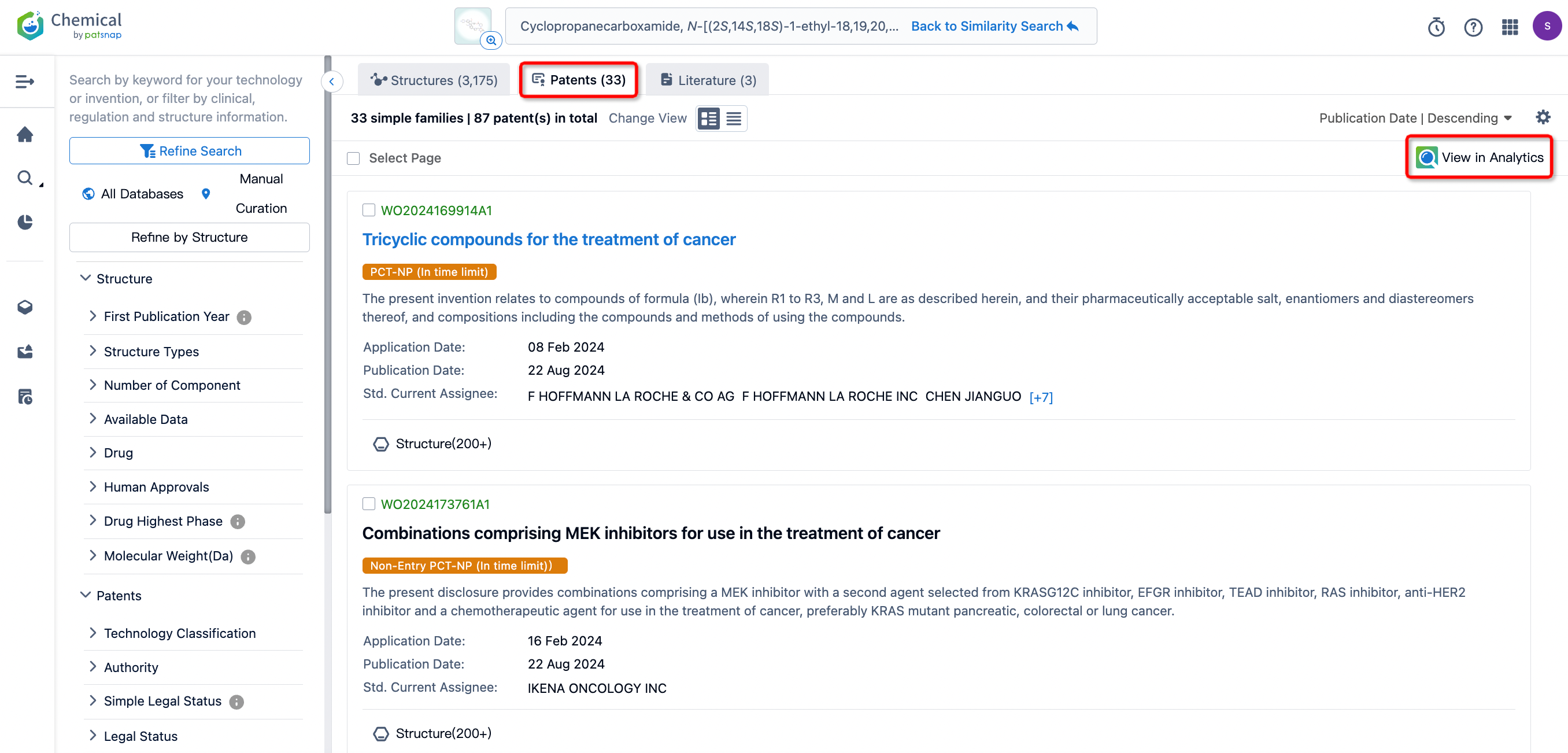
For a streamlined view of overlapping patent applications with competing companies, we can filter out applications from the original innovator, Revolution Medicines.
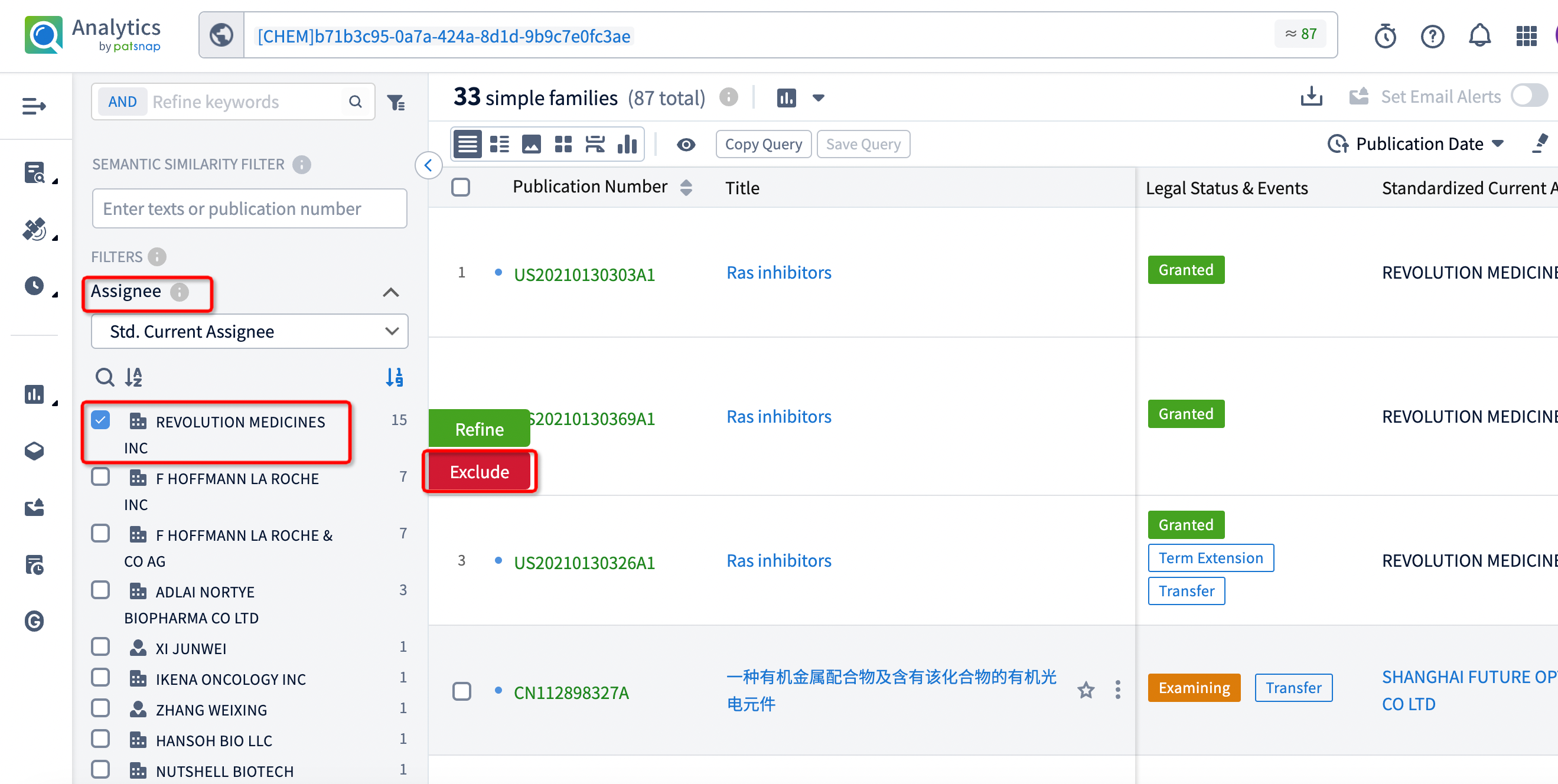
After filtering, 18 patents remain. By clicking on the publication number of each patent, the detailed information for each patent can be directly viewed.
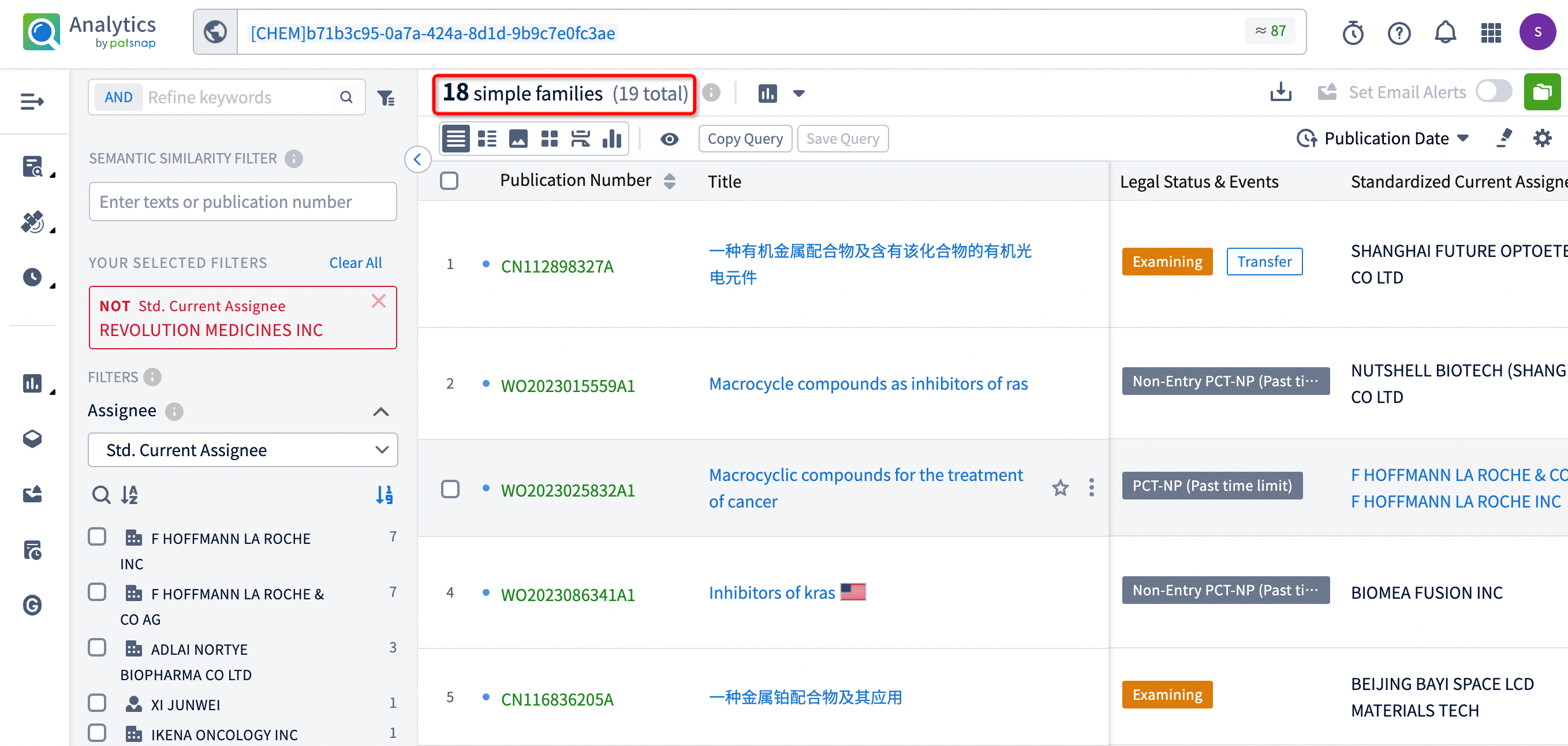
An examination of these patents reveals that the companies involved in patent applications with similar structures to RMC-6236 include Shanghai Future Optoetech, F. Hoffmann-La Roche, Biomea Fusion, Hansoh Biomedical, Adlai Nortye Biopharma, and Genfleet Therapeutics. Analyzing the specific compound structures from each company shows several structural similarities and overlaps among the compounds.
For instance, Genfleet’s patent WO2024153208A1 features compounds with a primary modification at the R5 substituent, where the original claim's coverage is particularly broad. Based on the advantageous molecules presented in its examples and the claim layout, the key modification in the R5 substituent involves a substituted propargyl group.
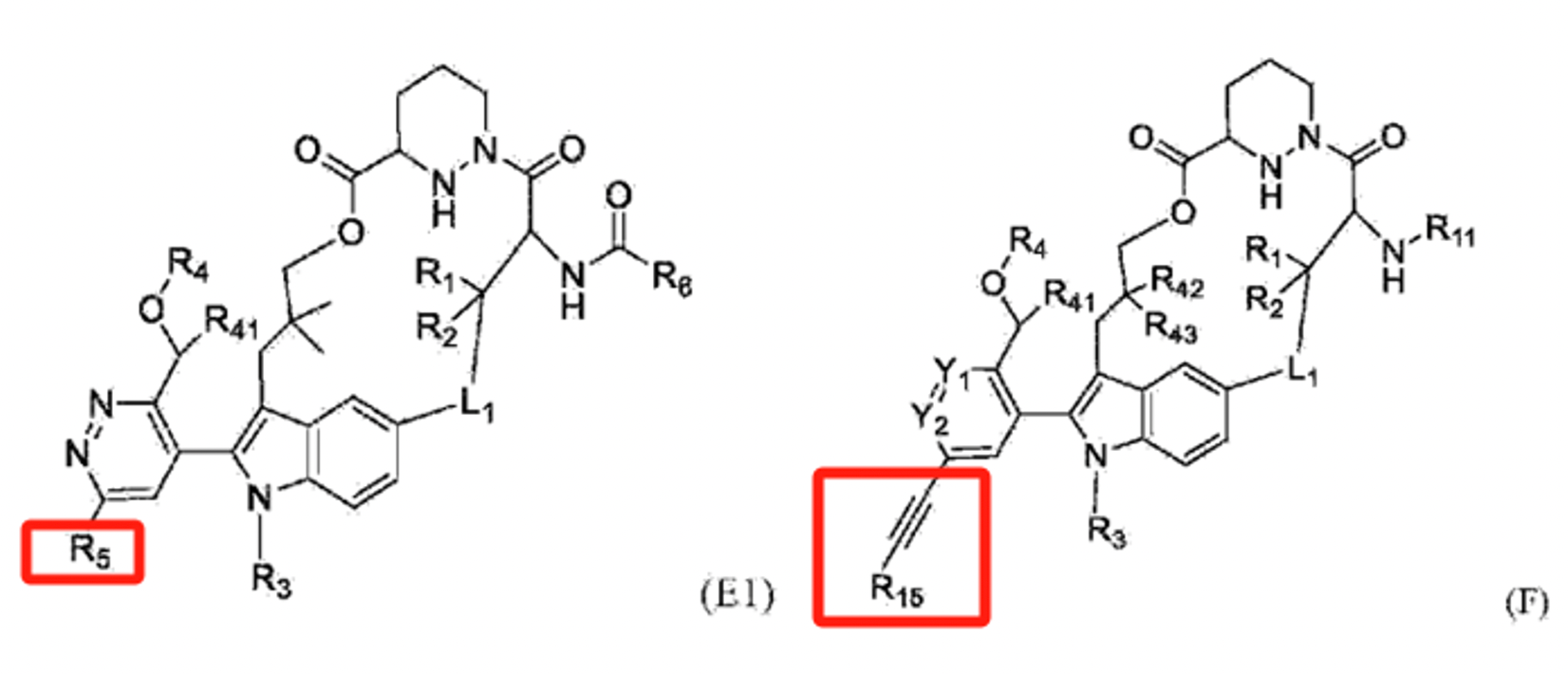
Similarly, in Adlai Nortye’s patent CN117903169A, the structural modification is also centered on this position. Examining the claim scope of both patents reveals considerable overlap, with Genfleet’s patent having a later filing date than Adlai Nortye’s. During the subsequent substantive examination stage, this is likely to impact the assessment of Genfleet’s patent’s novelty or inventiveness. Compared to the current extensive coverage, a narrower claim scope and sufficient evidence of inventiveness may be necessary for Genfleet to secure authorization.
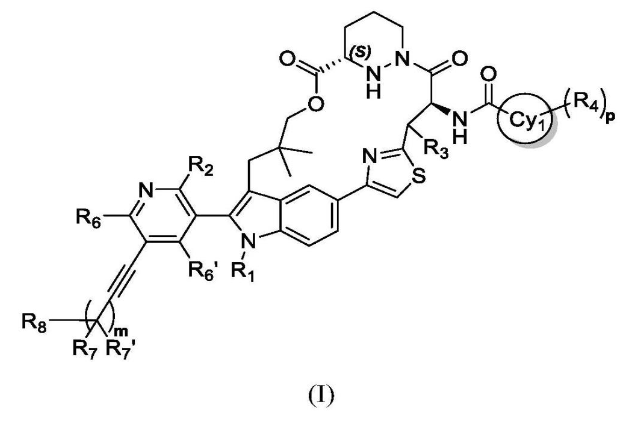

Likewise, Biomea Fusion’s patent WO2023086341A1 introduces structural modifications at the side chain, particularly with the inclusion of tetrahydropyrrole and propargyl groups.
Nutshell Biotech’s patent WO2023015559A1 also involves modifications at this position, disclosing L substituents that can be tetrahydropyrrole and W substituents preferably as propargyl. Among the specific compounds disclosed, tetrahydropyrrole and propargyl structures are also included. Although Nutshell Biotech’s application date precedes that of Biomea Fusion, the publication dates of the two patents are only three months apart, underscoring the intense competition in the pan-KRAS development field.
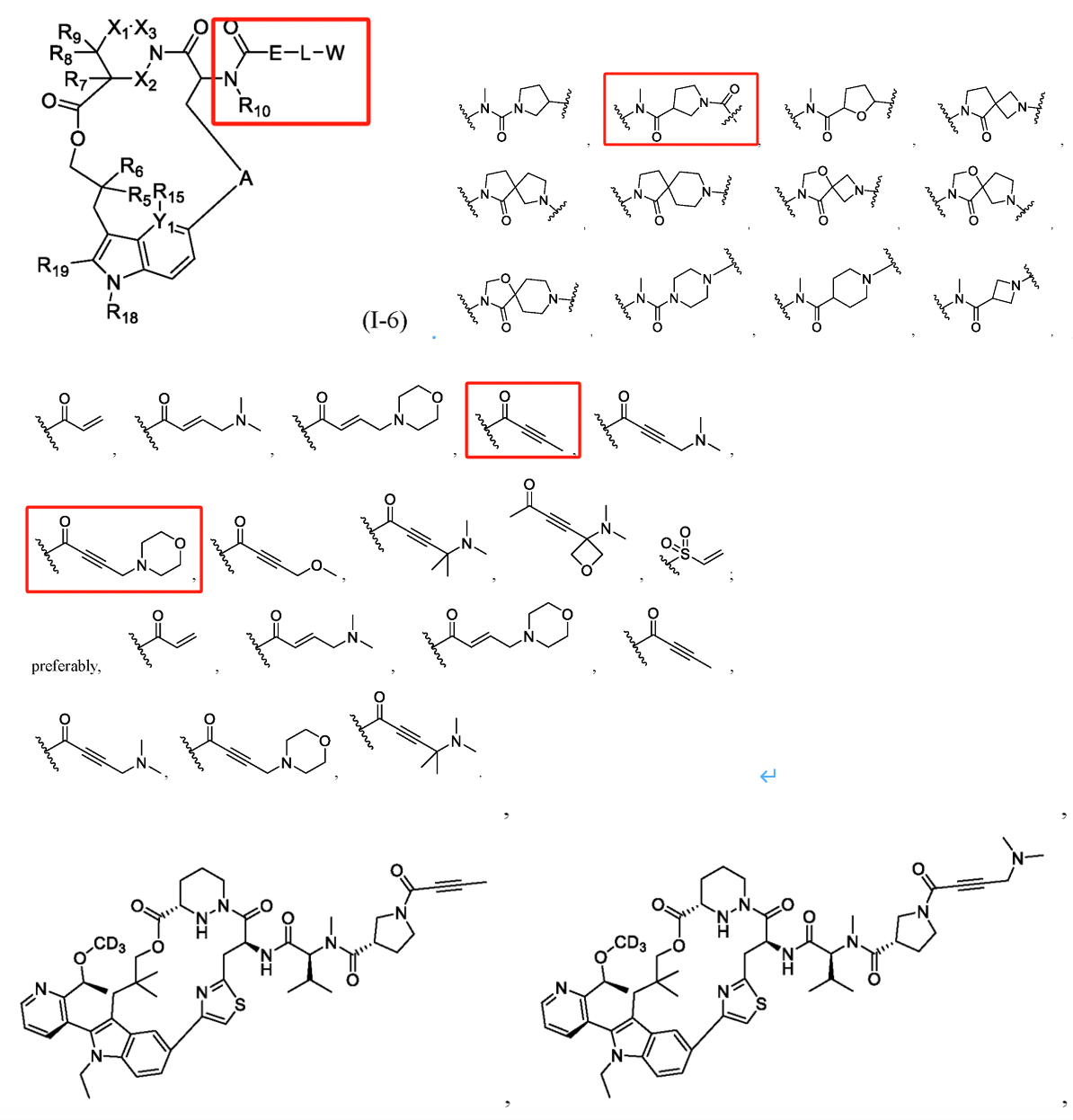
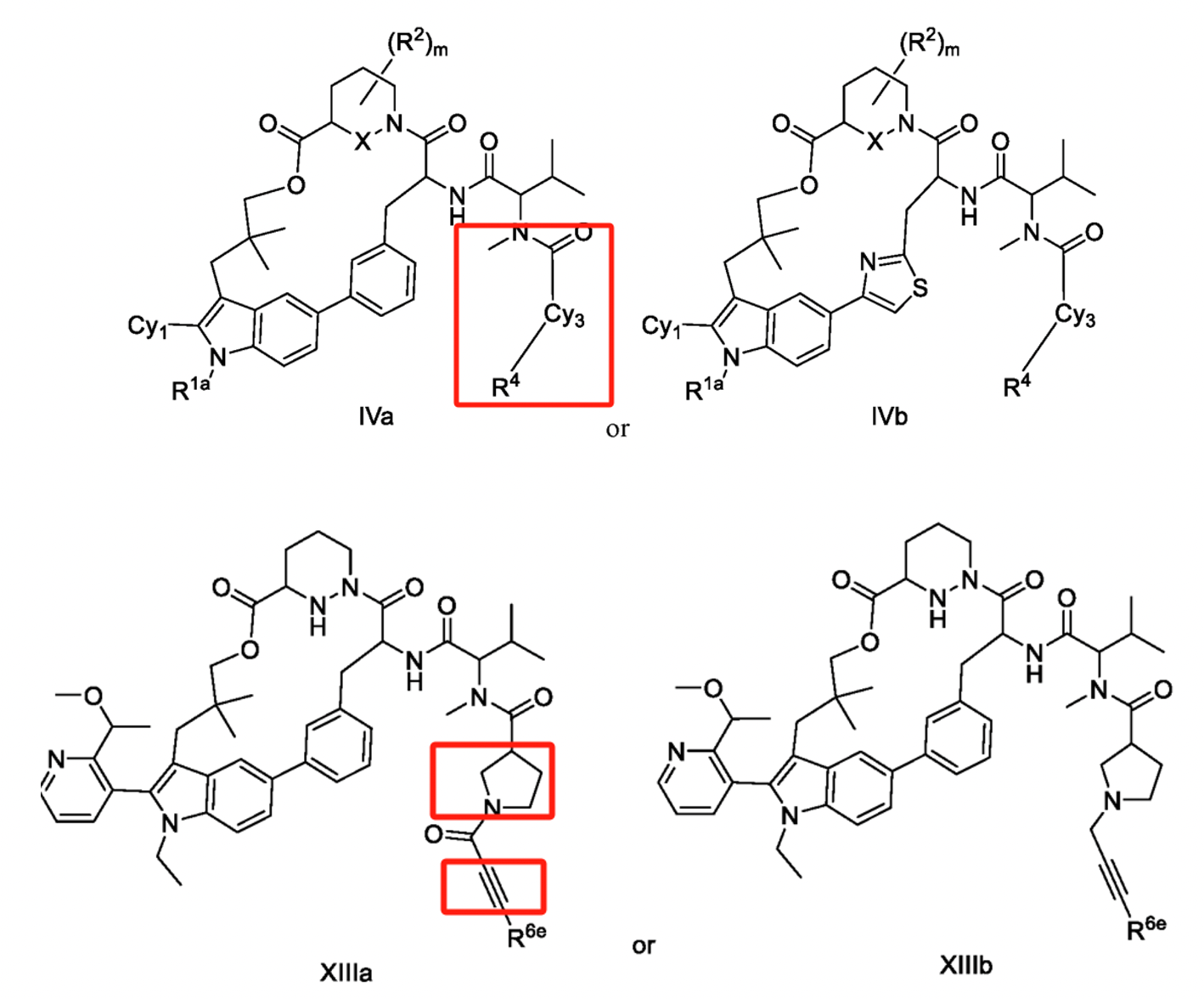
Similarly, in F. Hoffmann-La Roche’s patent WO2023025832A1 (with the Chinese family member CN117693509A), the compound modification also focuses on the side chain at R1. Compared to other companies, this patent has a notably narrower claim scope, and comparative experimental data provided in its examples confirm that the R1 substituent modification reduces clearance rates, enhancing human hepatocyte stability. In such a crowded field, refining smaller inventive concepts to ensure patentability is a promising strategy for patent positioning.
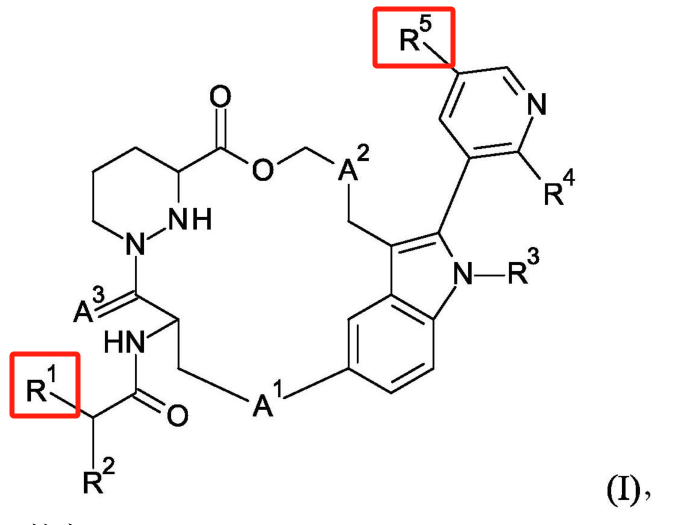
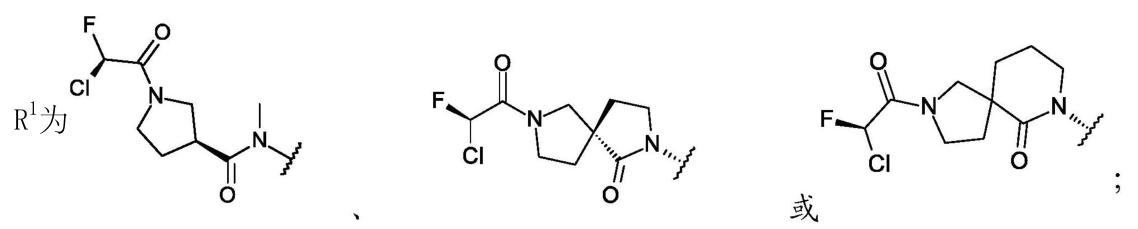
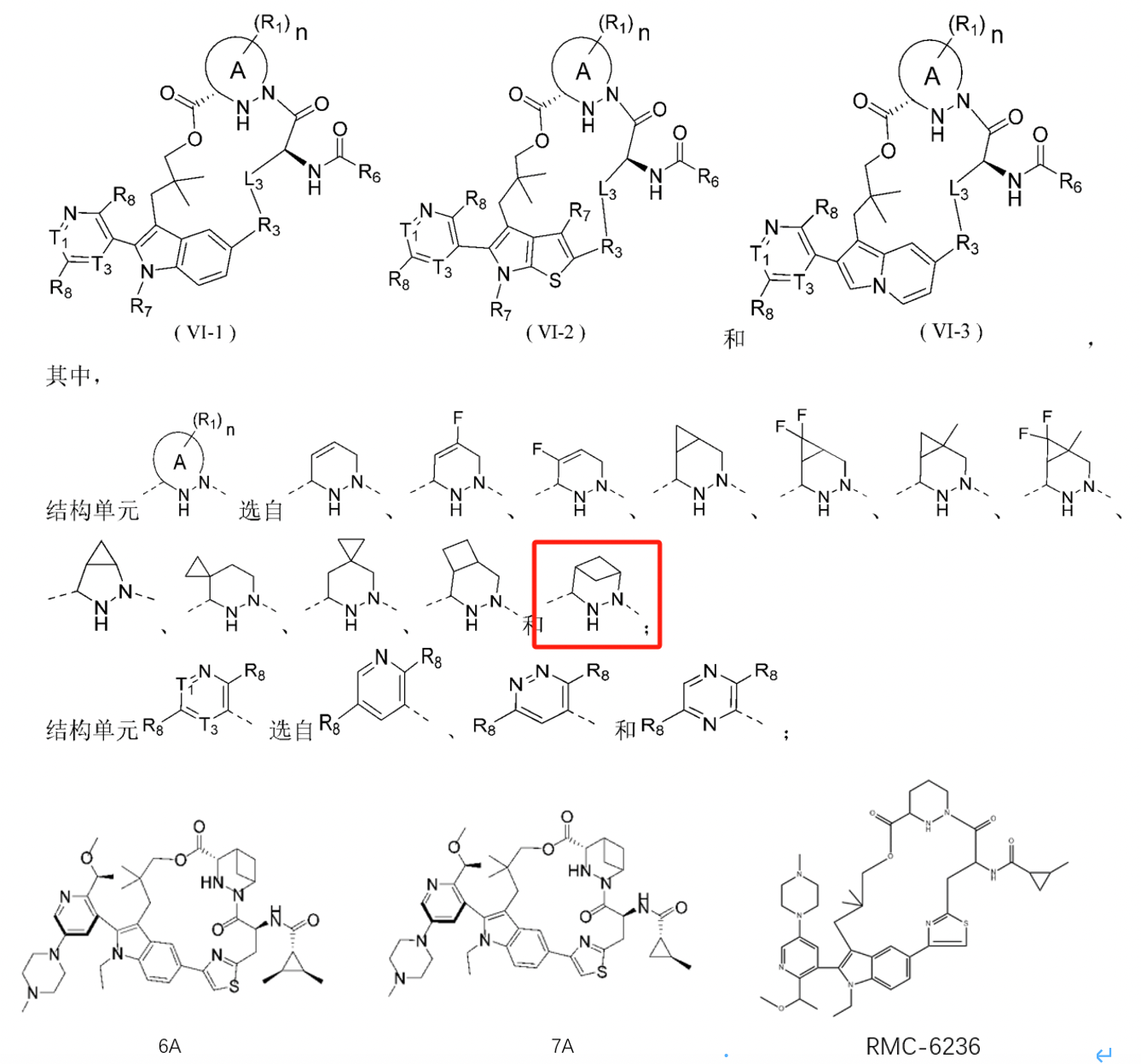
Distinct from the other companies, Medshine Discovery’s patent WO2024067857A1 involves modifications on ring A, particularly in the form of a bridged structure. There is no motivation in existing technologies for this modification on ring A, setting its structure apart and minimizing overlap risk, which likely improves the chances of patent approval. Compound 7A in the examples differs from RMC-6236 only in the ring A structure, yet pharmacokinetic studies show that compounds 6A and 7A exhibit high exposure levels and long half-lives, demonstrating favorable pharmacokinetic profiles. This highlights the appeal of pharmaceutical R&D, where a subtle structural modification can result in substantial activity differences.
The pan-KRAS field is in a rapid growth phase, with multiple new drugs under development and clinical trials underway, potentially offering more treatment options for patients with KRAS-mutant cancers. The core patents for Eli Lilly’s LY4066434, Jacobio’s JAB-23E73, and BeiGene’s BGB-53038 have not been disclosed, so it remains uncertain if there will be any patent conflicts with these pan-KRAS inhibitors.
The intense competition in the development of pan-KRAS inhibitors underscores the importance of conducting FTO (Freedom to Operate) analyses. Patsnap Chemical, with its powerful search capabilities and extensive data resources, enables seamless cross-referencing between chemical structures and drug information. This integration of chemical structure with patent, literature, and clinical trial data facilitates FTO searches, patent research for R&D, and can help us make more informed decisions in new drug development and intellectual property protection.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.