EU Commission Approves Pfizer's ABRYSVO™ for RSV Protection in Infants, Elderly via Maternal Vaccination
Pfizer Inc. disclosed today that ABRYSVO™, their dual-action respiratory syncytial virus (RSV) prefusion F (RSVpreF) vaccine, has been granted marketing approval by the European Commission. This will bolster protection for both infants via maternal immunization and senior citizens.
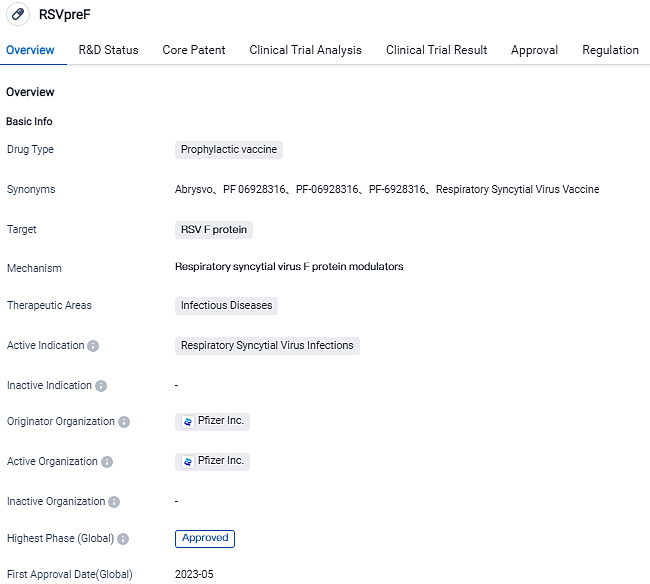
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Active defence from lower respiratory tract disease (LRTD) prompted by RSV in newborns up to six months of age, thanks to maternal vaccination during gestation.Active vaccination for individuals aged 60 and above to prevent LRTD induced by RSV.
"The endorsement of ABRYSVO in Europe signifies outstanding progress in our quest for effective RSV protection - a prevalent respiratory virus that might be severe or even fatal, specifically for infants and elderly people," stated Annaliesa Anderson, Ph.D., Senior VP and Director of Vaccine Research and Development at Pfizer.
"The overwhelming numbers of newly born, children, and adults hospitalized throughout Europe last year underscored the critical demand for protection against acute RSV incidents. The vaccine's approval for both elderly individuals and newborns via maternal immunization marks a major public health victory and we anticipate a considerable impact in the foreseeable future."
This marketing license follows the recent positive feedback from the Committee for Medicinal Products for Human Use. The license is effective in all 27 EU member countries, including Iceland, Liechtenstein, and Norway.
ABRYSVO is the pioneer approved vaccine specifically designed and tested for maternal immunization and now a single dose can be administered in the EU during the 24th to 36th weeks of pregnancy. Moreover, ABRYSVO has also been examined in adults aged 60 and above. The marketing approval also covers single-dose usage in this group.
RENOIR is an internationally conducted, randomized, obscured-observer, placebo-regulated trial developed to measure the effectiveness, immunological response, and safety of a singular dosage of the vaccine in individuals aged 60 and over.
MATISSE is an international, randomized, concealed-observer, placebo-regulated trial established to examine the efficiency, safety, and immunological reaction of RSVpreF against LRTD and severe LRTD caused by RSV in babies born to healthy vaccinated individuals during gestation.Burden of Disease in Europe
RSV is an infectious virus that is frequently responsible for respiratory ailments across the globe. It has the potential to damage the lungs and respiratory tract of a person it infects, which could lead to serious illness or mortality. In the EU, around 245,000 ancillary hospital admissions each year pertain to RSV in children below the age of five, with the majority being recorded in children under one year old. The pathology load for the elderly is also not negligible. On an annual basis, the virus triggers about 270,000 hospital stays and nearly 20,000 fatalities among those aged 60 and above.
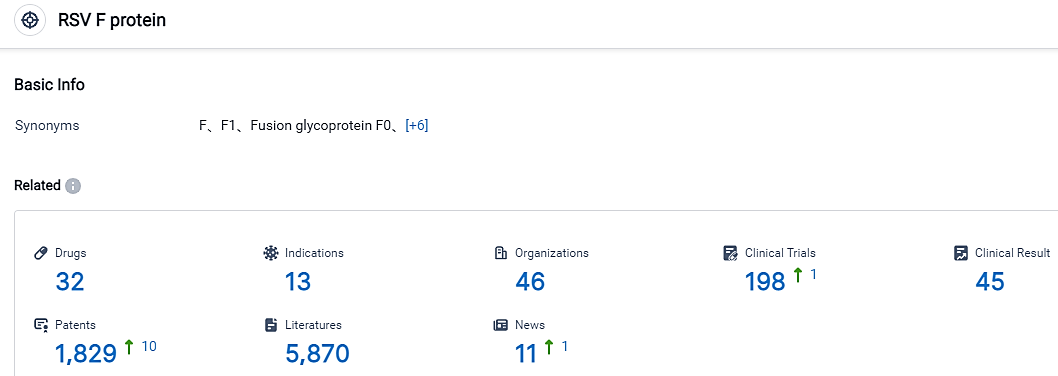
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 5, 2023, there are 32 investigational drugs for the RSV F protein target, including 13 applicable indications, 46 R&D institutions involved, with related clinical trials reaching 198, and as many as 1829 patents.
Currently, Pfizer stands as the sole entity that offers a Respiratory Syncytial Virus (RSV) vaccine to safeguard the elderly and ensure infant protection through maternal vaccination. A few days ago, the United States' Food and Drug Administration sanctioned the usage of RSVpreF, dubbed ABRYSVO, aimed at preventing lower respiratory tract disease (LRTD) and its severe variant in newborns up to six months old, employing the active vaccination of expectant patients.
This approval goes after the nod given by the FDA in May 2023 for ABRYSVO to be used in guarding against LRTD stemming from RSV in individuals above 60 years old. Pfizer has kick-started two supplementary clinical trial assessments of ABRYSVO. One of these trials targets children more susceptible to RSV-related illnesses, spanning the ages of two to just below 18 years. Additionally, Pfizer is strategizing on initiating post-approval research and monitoring schemes to expand the understanding of the vaccine's safety profile.