Is Brincidofovir approved by the FDA?
Brincidofovir, marketed under the brand name Tembexa, is an antiviral medication classified under purine nucleosides. Tembexa (brincidofovir) was approved by the U.S. Food and Drug Administration (FDA) on June 4, 2021. This approval provides a crucial tool in the event of a smallpox outbreak, given the potential use of the virus as a bioterrorism agent.
Indications and Usage
Brincidofovir is prescribed for the treatment of smallpox. It is available in both tablet and oral suspension forms. The medication is specifically indicated for:
- Adults and children weighing 48 kg or more: 200 mg (two 100 mg tablets) once a week for two doses (on Days 1 and 8).
- Children weighing less than 48 kg: Dosage is based on body weight and is administered as an oral liquid.
Administration
Brincidofovir should be taken exactly as directed by a healthcare provider. The tablet form can be taken with or without a low-fat meal, while the oral liquid is best taken on an empty stomach. For those unable to swallow the liquid, it can be administered through a nasogastric or gastrostomy tube.
Warnings and Precautions
Increased Mortality Risk: There is an increased risk of mortality when brincidofovir is used for longer durations, as observed in a 24-week clinical trial.
Liver and Gastrointestinal Issues: Brincidofovir may cause serious liver problems and gastrointestinal issues. Symptoms such as dark urine, nausea, vomiting, stomach pain, or yellowing of the skin and eyes should be reported to a healthcare provider immediately.
Pregnancy and Infertility: The medication can harm an unborn baby and may cause birth defects. Both male and female patients should use effective birth control during treatment and for a period after the last dose. Additionally, the drug may lead to infertility in men.
Side Effects
Common side effects include:
- Diarrhea
- Nausea
- Vomiting
- Stomach pain or cramps
- Swelling of extremities
Less common side effects can include skin rashes, changes in taste, and muscle weakness. Any severe or unusual symptoms should prompt immediate medical attention.
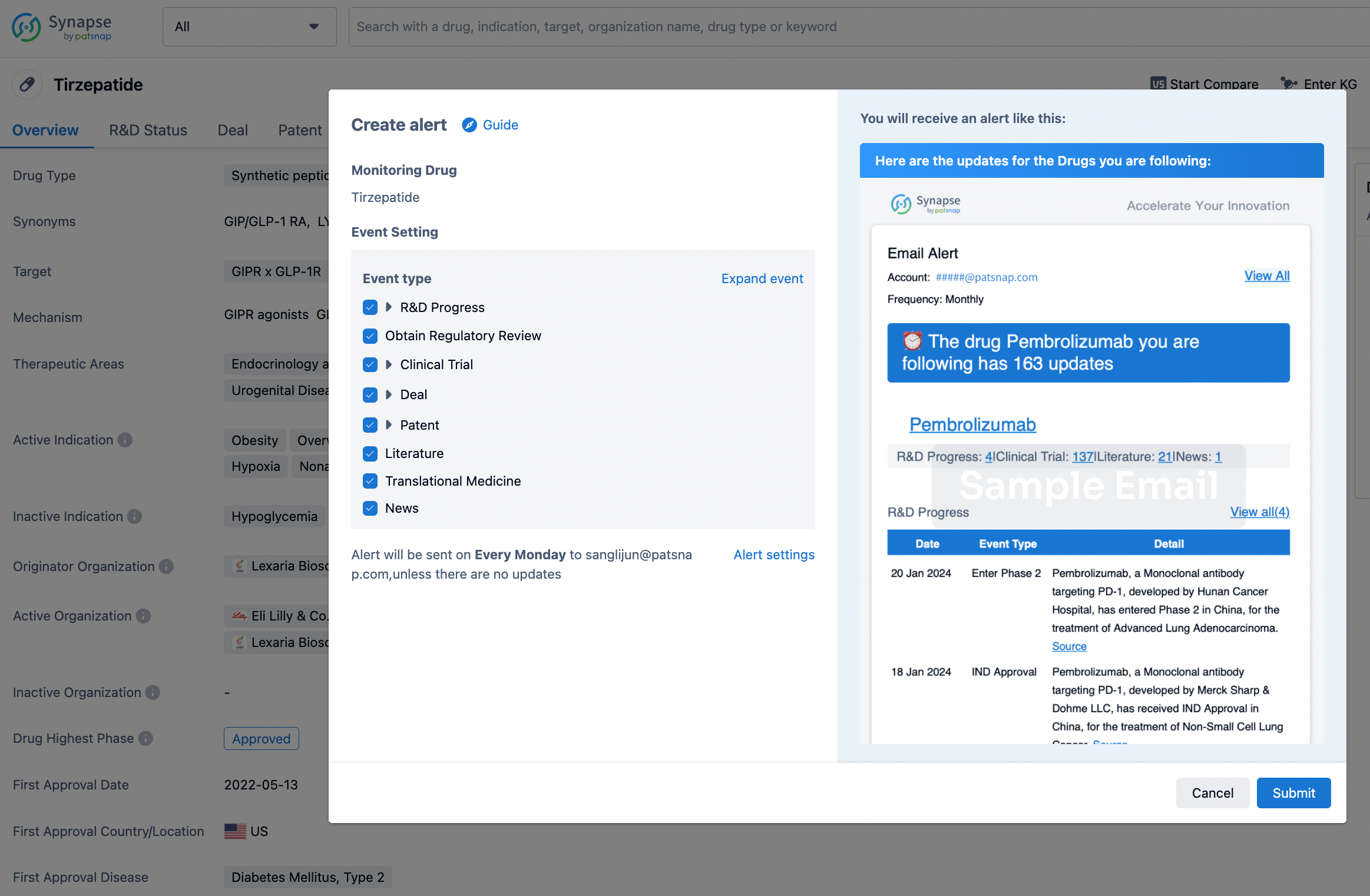
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!