Is Casirivimab/Imdevimab approved by the FDA?
Casirivimab and Imdevimab, known by the brand name Regen-Cov, are monoclonal antibodies used as a combination therapy for the treatment and prevention of COVID-19. They belong to the drug class of antiviral combinations and are administered via injection.
Casirivimab and Imdevimab received Emergency Use Authorization (EUA) from the FDA on November 21, 2020. This EUA allowed their use for treating mild to moderate COVID-19 in patients who are at high risk for progressing to severe COVID-19, including hospitalization or death.
Use and Effectiveness
These antibodies are designed to target the SARS-CoV-2 virus that causes COVID-19. Regen-Cov can be used for:
- Treatment of COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive COVID-19 test results who are at high risk for progressing to severe COVID-19.
- Post-exposure prophylaxis in individuals who have been exposed to COVID-19 and are at high risk of severe disease.
However, due to the emergence and high frequency of the Omicron BA.2 sub-variant, which has shown resistance to these antibodies, the FDA has paused the authorization of Casirivimab and Imdevimab in the US as of early 2022. This means they are not currently recommended for use in any region of the US until further notice.
Administration
Regen-Cov is administered either through intravenous infusion or subcutaneous injection. The infusion process usually takes 20 to 50 minutes or longer, while the subcutaneous injection involves 2 to 4 separate injections into different areas of the body. Monitoring for allergic reactions is essential during and after administration.
Side Effects
Common Side Effects:
- Injection site reactions (redness, swelling, pain)
- Fever, chills
- Nausea, vomiting, diarrhea
- Headache
- Muscle or joint pain
- Increased or decreased heart rate
Serious Side Effects:
- Severe allergic reactions (anaphylaxis)
- Infusion-related reactions (fever, chills, nausea, headache, shortness of breath)
- Symptoms of infection (fever, chills, cough, rash, swelling, diarrhea, pain or burning when urinating)
Warnings and Precautions
- Casirivimab and Imdevimab should not be used in individuals who are hospitalized due to COVID-19 or require oxygen therapy due to COVID-19.
- It may not be effective against certain variants of SARS-CoV-2, particularly the Omicron variant.
- Patients with a history of severe allergic reactions to any component of the therapy should avoid its use.
Conclusion
While Casirivimab and Imdevimab were authorized for emergency use by the FDA to treat and prevent COVID-19, their current use is paused due to the reduced effectiveness against the prevalent Omicron variant. It remains a significant treatment option for earlier variants and in specific scenarios, pending further updates from health authorities.
How to obtain the latest development progress of all drugs?
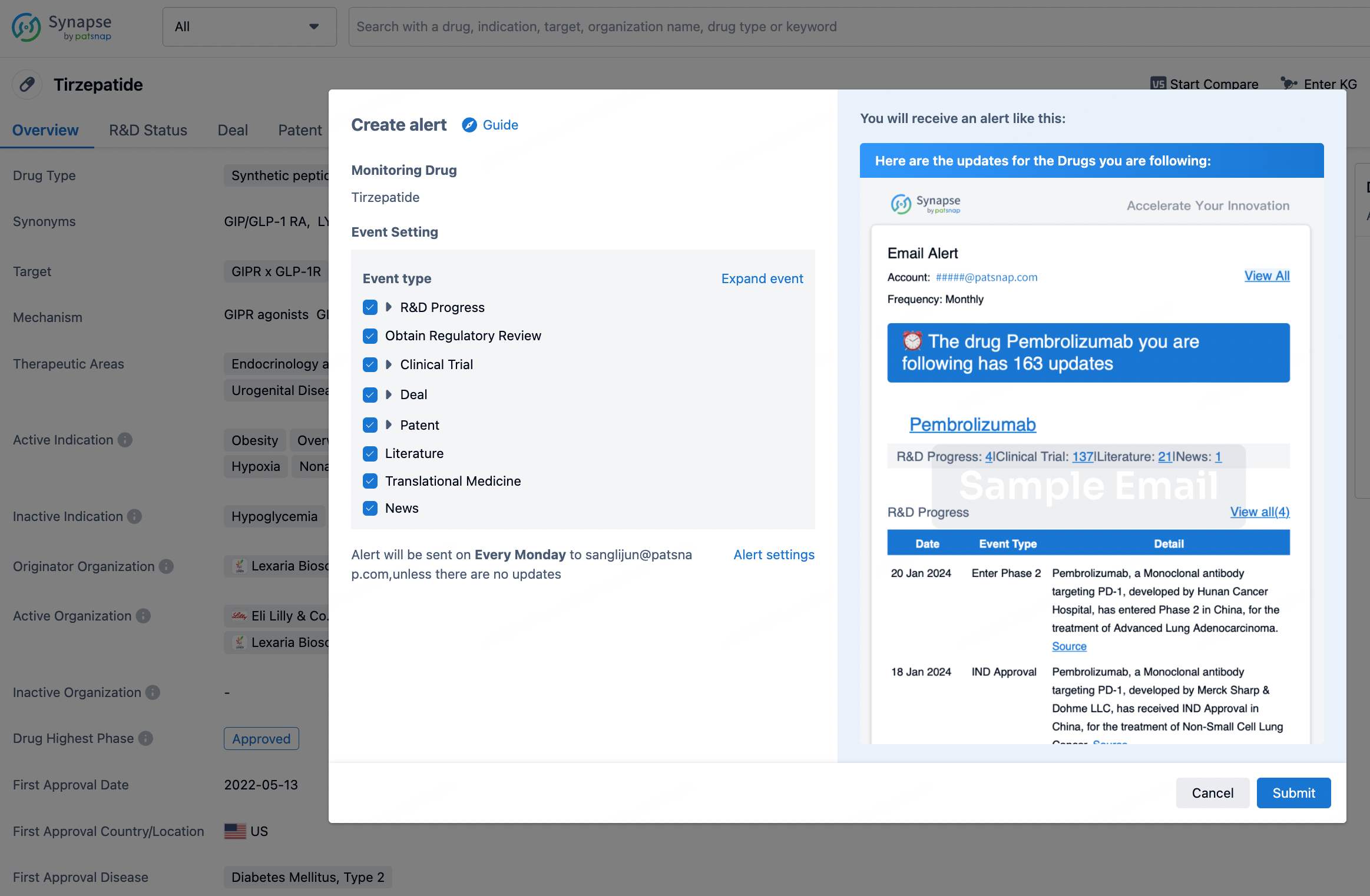
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!