Is Diroximel Fumarate approved by the FDA?
Yes, diroximel fumarate is FDA approved. The U.S. Food and Drug Administration (FDA) approved diroximel fumarate, marketed under the brand name Vumerity, on October 29, 2019. This medication is used to treat relapsing forms of multiple sclerosis (MS) in adults.
What is Diroximel Fumarate?
Diroximel fumarate is an oral delayed-release capsule that belongs to the drug class of selective immunosuppressants. It is specifically designed for the treatment of multiple sclerosis (MS), which includes:
- Clinically isolated syndrome
- Relapsing-remitting disease
- Active secondary progressive disease
How Does Diroximel Fumarate Work?
Diroximel fumarate helps to manage MS by modulating the immune system, although its exact mechanism in the treatment of MS is not fully understood. It is thought to work by reducing inflammation and preventing nerve damage.
Indications and Usage
Diroximel fumarate is prescribed for adults with relapsing forms of multiple sclerosis. It is not approved for use in children under 18 years of age.
Dosage and Administration
The recommended dosage of diroximel fumarate for adults with MS is as follows:
- Initial Dose: 231 mg taken orally twice a day for the first 7 days.
- Maintenance Dose: 462 mg taken orally twice a day thereafter.
Patients are advised to swallow the capsules whole without crushing, chewing, breaking, or opening them. To minimize gastrointestinal side effects, it is recommended to avoid high-fat, high-calorie meals when taking this medication. Each meal or snack should not exceed 700 calories or 30 grams of fat.
Side Effects
Common side effects of diroximel fumarate include:
- Indigestion, nausea, vomiting, stomach pain
- Skin rash, itching, redness
- Diarrhea
- Flushing (sudden warmth, redness, or tingling feeling)
Serious side effects may also occur, including:
- Liver problems (loss of appetite, stomach pain, tiredness, dark urine, jaundice)
- Serious brain infection (symptoms related to speech, thought, vision, or muscle movement)
- Signs of infection (fever, chills, cough, shortness of breath, headache, neck stiffness, increased sensitivity to light, nausea, vomiting)
Warnings and Precautions
- Allergic Reactions: Patients who have had a severe allergic reaction to diroximel fumarate or dimethyl fumarate should not use this medication.
- Liver and Kidney Disease: Patients with a history of liver or kidney disease should inform their healthcare provider before starting treatment.
- Pregnancy and Breastfeeding: The effects of diroximel fumarate during pregnancy and breastfeeding are not well known. Patients should discuss potential risks and benefits with their healthcare provider.
Drug Interactions
Certain medications may interact with diroximel fumarate, potentially affecting its efficacy or increasing side effects. Notable interactions include:
- Rifampin
- St. John's wort
- Seizure medications (e.g., carbamazepine, phenobarbital)
- Other medications that affect liver enzymes
Patients should provide a complete list of their current medications to their healthcare provider to avoid potential interactions.
Conclusion
Diroximel fumarate (Vumerity) is a significant option for the treatment of relapsing forms of multiple sclerosis, approved by the FDA on October 30, 2019. It offers a valuable treatment option for adults with MS, helping to manage the disease and improve quality of life. As with any medication, it is crucial to follow the prescribed dosage and guidelines and to consult with a healthcare provider to manage potential side effects and interactions effectively.
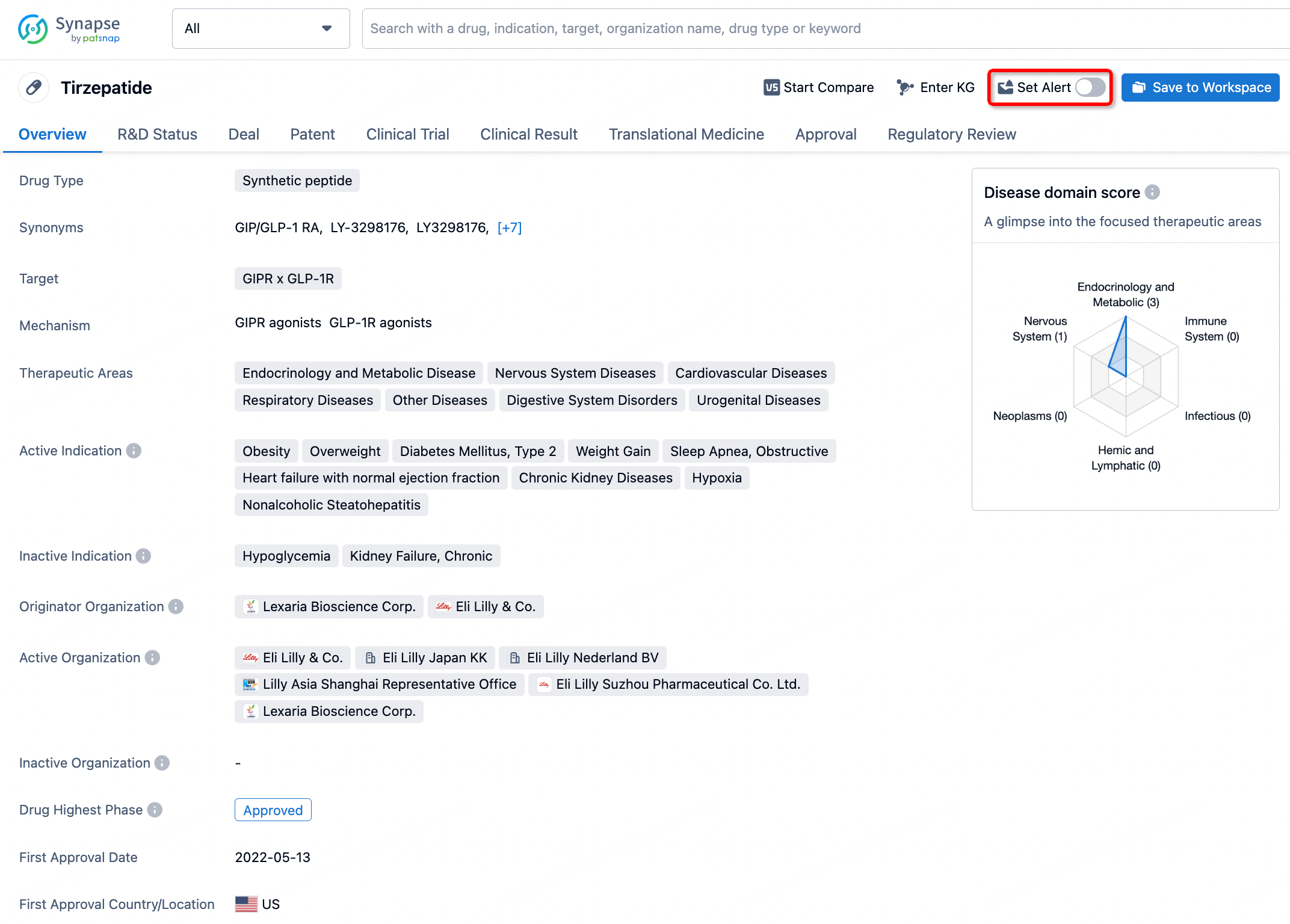
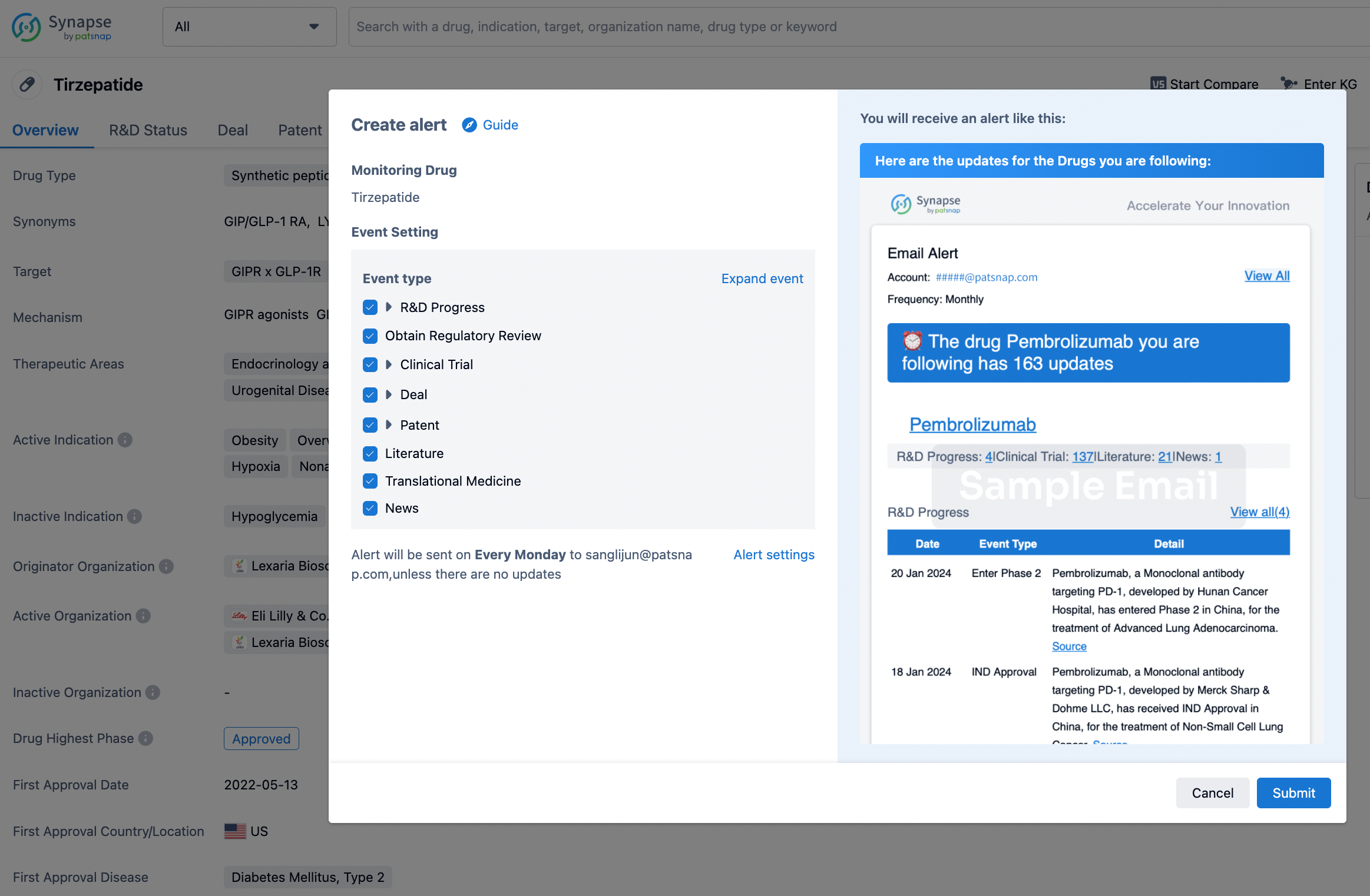
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!