Day One Advances Clinical Pipeline with Innovative ADC Targeting PTK7 in Solid Tumors
Day One Biopharmaceuticals, a biopharmaceutical company at the commercial stage focused on creating and marketing targeted treatments for individuals of all ages with severe illnesses, revealed it has forged an exclusive licensing deal with MabCare Therapeutics for MTX-13, a new ADC that targets protein-tyrosine kinase 7 (PTK7). According to the Agreement's provisions, Day One holds the exclusive global rights, except in Greater China, to develop, produce, and market MTX-13.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
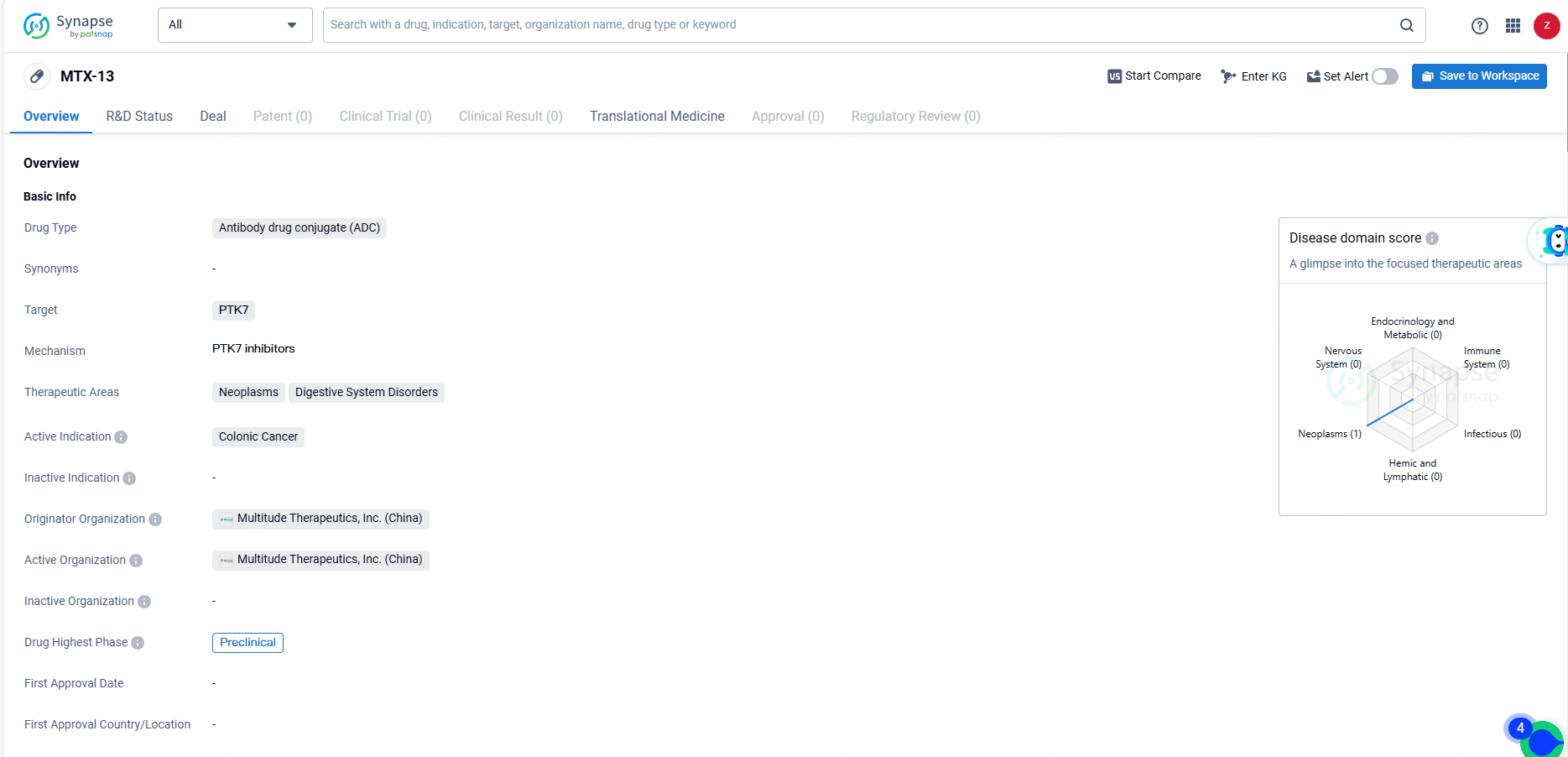
In April 2024, the U.S. Food and Drug Administration approved the investigational new drug application for MTX-13, which will subsequently be known as DAY301. Pre-clinical research indicated that DAY301 has antitumor properties across a variety of solid tumors.
“Our goals for 2024 include the successful introduction of OJEMDA™ (tovorafenib), the progression of our current projects, and the expansion of our pipeline by acquiring clinical-stage assets that could revolutionize outcomes for cancer patients of all ages,” stated Jeremy Bender, Ph.D., the CEO of Day One. “We are thrilled by the potential of DAY301 and are confident that we have the right team to fully develop this program.”
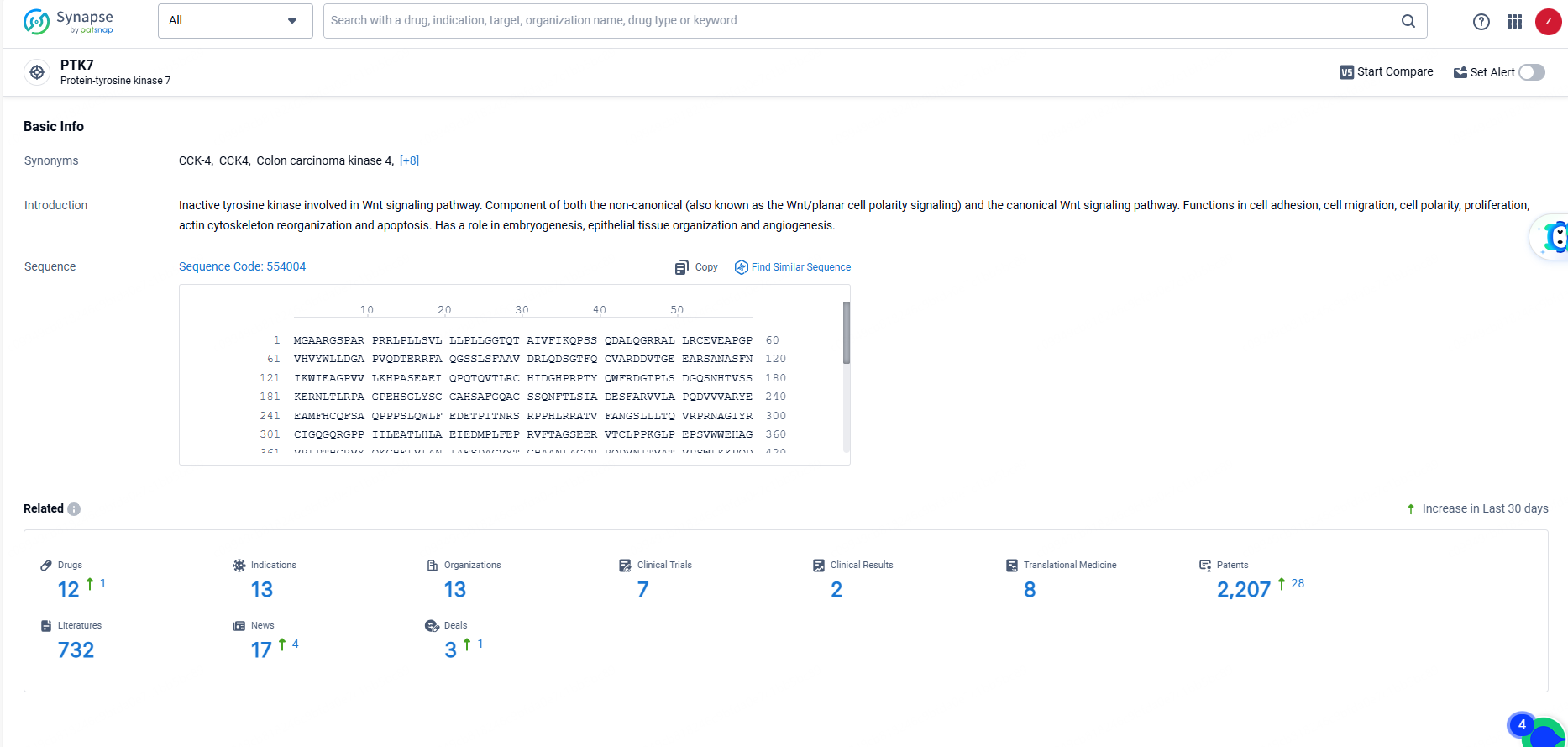
DAY301 is designed to target PTK7, a highly conserved, catalytically inactive transmembrane protein that is overexpressed in various adult cancers such as esophageal, ovarian, lung, and endometrial cancer, as well as in pediatric cancers like neuroblastoma, rhabdomyosarcoma, and osteosarcoma. The limited expression of PTK7 in normal tissues or organs makes it an excellent target for therapeutic development.
“The incorporation of DAY301 into our pipeline aligns with our mission to advance both pediatric and adult medicines in areas of high unmet need with equal priority,” said Dr. Samuel Blackman, co-founder and head of research and development at Day One. “We believe the linker-payload technology of DAY301 can overcome the limitations of previous PTK7-targeted ADCs, positioning it as a potentially first-in-class drug against a clinically validated target. We are enthusiastic about bringing this program into Day One and aim to begin clinical trials in the upcoming months.”
As part of the licensing agreement, MabCare will receive $55 million initially and is eligible for up to $1.152 billion in additional development, regulatory, and commercial milestones, along with low-to-mid single-digit royalties on net sales outside of Greater China. Day One anticipates enrolling the first patient in the Phase I study in late 2024 or early 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 8 investigational drugs for the PTK7 target, including 47 indications, 25 R&D institutions involved, with related clinical trials reaching 63, and as many as 795 patents.
MTX-13 drug targets PTK7 and is currently in the preclinical phase of development, with a focus on neoplasms and digestive system disorders. As a potential treatment for a significant medical condition, the progress of MTX-13 in preclinical studies may hold promise for the future of colonic cancer therapy.