Is Donislecel approved by the FDA?
Donislecel, branded as Lantidra, was approved by the FDA on June 28, 2023. It represents a significant development in the treatment of type 1 diabetes, particularly for patients who struggle with severe hypoglycemia despite intensive diabetes management.
Uses for Donislecel
Donislecel is indicated for adults with type 1 diabetes who cannot achieve target HbA1c levels due to repeated severe hypoglycemia. This therapy is used in conjunction with immunosuppression to prevent islet graft rejection. However, its use is not recommended for patients who can manage their diabetes well with insulin therapy or those who can prevent severe hypoglycemic events through intensive diabetes management.
Administration and Dosage
Donislecel is administered as a suspension of islet cells infused into the hepatic portal vein. The dosage varies based on the number of islets, with the initial infusion requiring a minimum of 5,000 equivalent islet number (EIN) per kg of the patient's body weight. Subsequent infusions may be needed if insulin independence is not achieved within a year or is lost after achieving it.
Safety and Precautions
While Donislecel offers a promising treatment option, it carries risks associated with the infusion procedure and long-term immunosuppression. Patients are at increased risk of infections, malignancies, and other serious adverse reactions due to immunosuppression. It is crucial to monitor portal pressure, blood glucose levels, and signs of infection during and after the procedure. The safety of Donislecel in patients with liver disease, renal failure, or those who have received a renal transplant has not been established.
Efficacy
In clinical studies involving 30 patients, Donislecel showed promising results, with 25 patients achieving insulin independence. Among these, some maintained independence for over five years, demonstrating the potential long-term benefits of the therapy.
Conclusion
Donislecel, or Lantidra, received FDA approval on June 28, 2023, as a cellular therapy for specific adults with type 1 diabetes experiencing severe hypoglycemia. While it offers significant benefits, the associated risks necessitate careful patient selection and monitoring. This approval marks a notable advancement in diabetes treatment, providing a new option for those struggling with severe hypoglycemia despite intensive management efforts.
How to obtain the latest development progress of all drugs?
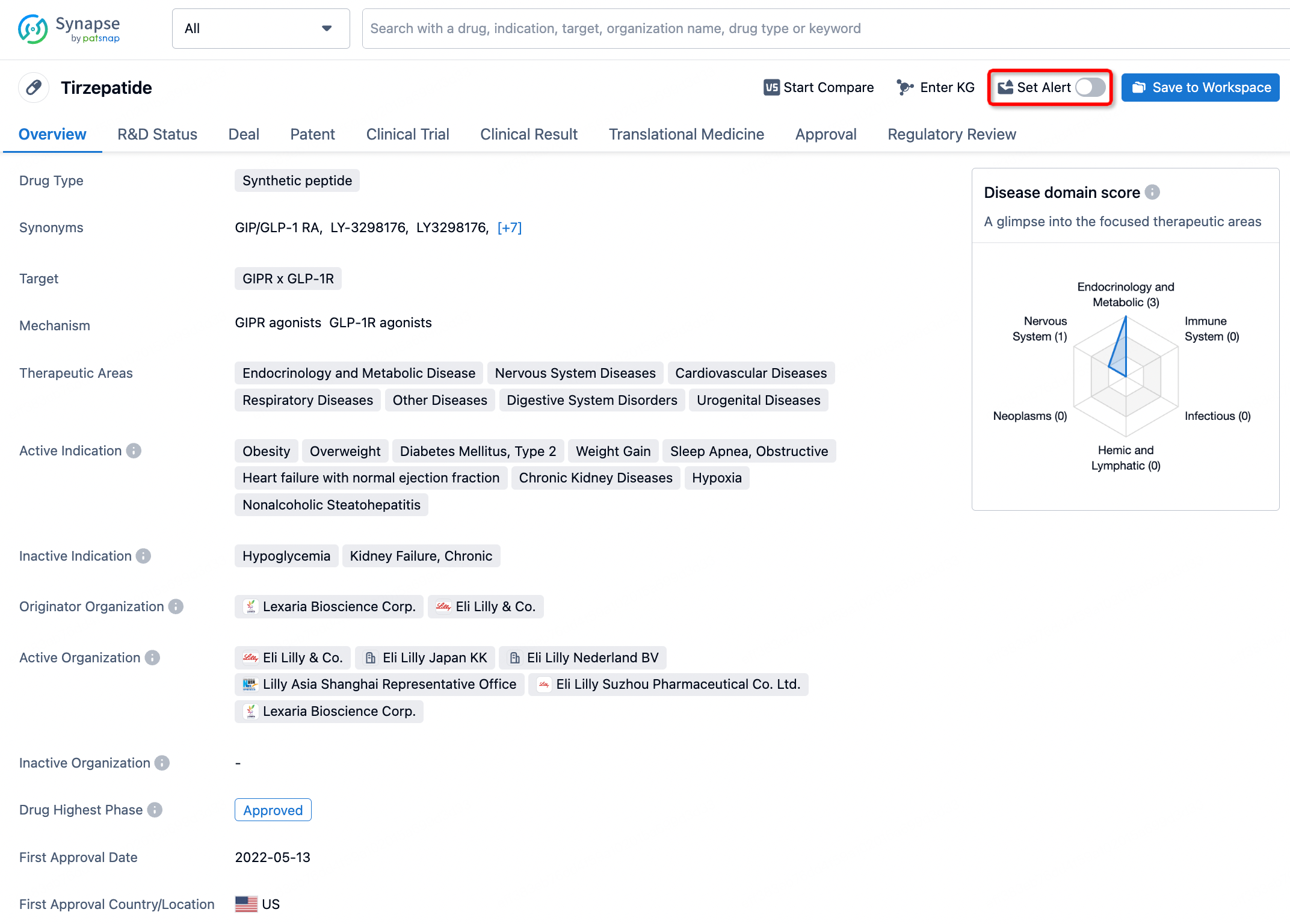
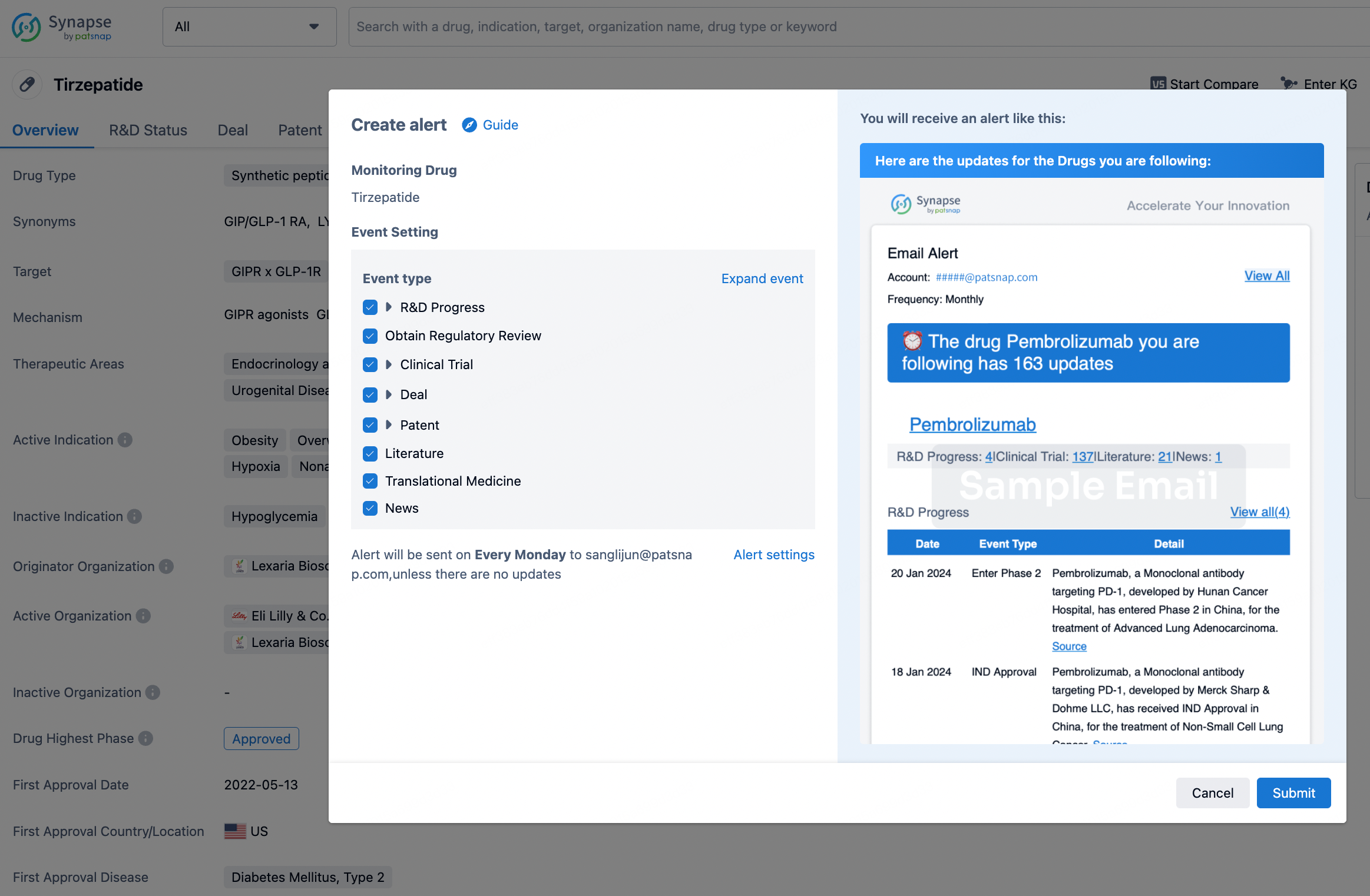
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!