Is Givosiran approved by the FDA?
Yes, Givosiran, marketed under the brand name Givlaari, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Givosiran on November 20, 2019, for the treatment of acute hepatic porphyria (AHP) in adults.
What is Givosiran?
Givosiran is a medication used to treat acute hepatic porphyria (AHP), a rare genetic disorder that can cause severe and potentially life-threatening attacks on the nervous system. These attacks can result in symptoms such as severe abdominal pain, nausea, vomiting, numbness, weakness, and mental status changes. AHP is more common in women during their child-bearing years.
Indications and Usage
Givosiran is indicated for the treatment of adults with acute hepatic porphyria. It helps to reduce the frequency and severity of attacks associated with this condition.
Dosage and Administration
Givosiran is administered as a subcutaneous injection, usually given once per month. The dosage is based on the patient's actual body weight:
- Usual Dose: 2.5 mg/kg administered subcutaneously once a month.
- Administration should be performed by a healthcare professional.
Side Effects
Common Side Effects
- Nausea
- Pain, itching, rash, discoloration, or swelling at the injection site
Serious Side Effects
Some side effects may necessitate delaying or permanently discontinuing treatment. These include:
- Allergic reactions such as hives, difficulty breathing, and swelling of the face, lips, tongue, or throat.
- Liver function abnormalities, requiring frequent blood tests to monitor liver health.
- Potential kidney function issues.
Warnings and Precautions
- Blood Tests: Frequent blood tests are necessary to monitor liver function. Kidney function should also be checked regularly.
- Pregnancy and Breastfeeding: It is not known whether Givosiran can harm an unborn baby. However, the risks of untreated acute hepatic porphyria during pregnancy may outweigh potential risks to the baby. It is also not clear if Givosiran passes into breast milk, so breastfeeding mothers should consult their healthcare provider.
Interactions
Patients should inform their healthcare provider about all medications they are currently taking, including prescription drugs, over-the-counter medicines, vitamins, and herbal supplements, as some drugs can affect the blood levels of Givosiran or its efficacy.
Conclusion
Givosiran (Givlaari) is an FDA-approved treatment for acute hepatic porphyria (AHP) in adults. Approved in November 2019, it offers a significant advancement in managing this rare and debilitating condition by reducing the frequency and severity of porphyria attacks. Patients should closely follow their healthcare provider's instructions and undergo regular monitoring to ensure the safe and effective use of this medication.
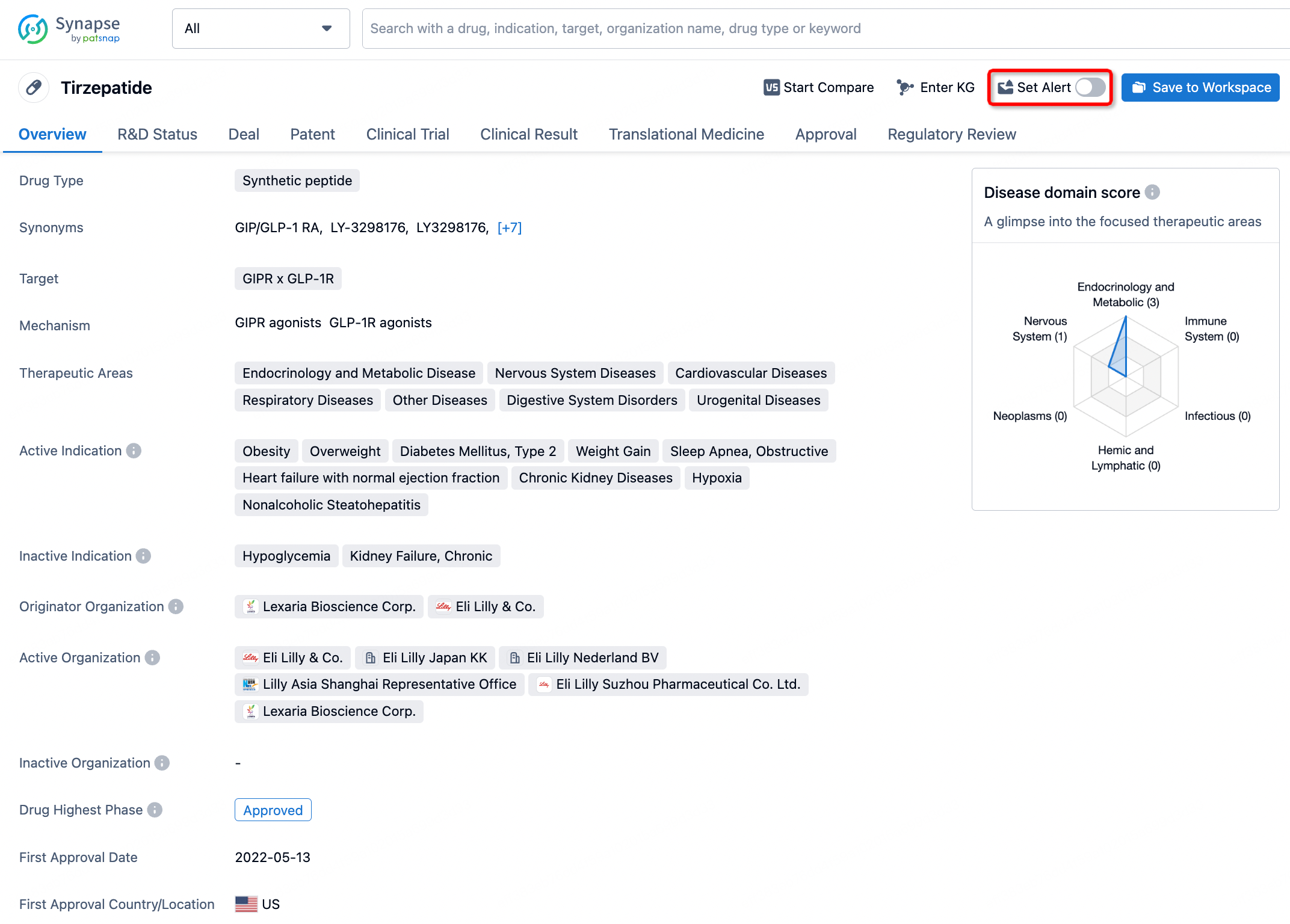
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!