Is Inebilizumab approved by the FDA?
Yes, inebilizumab (brand name: Uplizna) is FDA approved. The U.S. Food and Drug Administration (FDA) approved inebilizumab on June 11, 2020.
What is Inebilizumab?
Inebilizumab is an intravenous medication used to treat neuromyelitis optica spectrum disorder (NMOSD) in adults who are positive for the anti-aquaporin-4 (AQP4) antibody. NMOSD, also known as Devic's disease, is a central nervous system disorder where the immune system attacks the optic nerves and spinal cord, leading to symptoms such as vision loss, paralysis, and severe pain.
How Does Inebilizumab Work?
Inebilizumab is a monoclonal antibody that selectively targets and depletes B cells expressing CD19. By reducing the number of these B cells, inebilizumab helps to lower the risk of relapses and manage the symptoms of NMOSD.
Usage and Administration
Inebilizumab is administered via intravenous infusion. The initial treatment consists of two 300 mg infusions given two weeks apart. After the initial doses, a maintenance dose of 300 mg is administered every six months.
Pre-treatment and Monitoring:
- Patients are pre-medicated with corticosteroids, antihistamines, and antipyretics to prevent infusion reactions.
- They are closely monitored for at least one hour post-infusion for any adverse reactions.
Precautions and Considerations
Before Treatment:
- Ensure all vaccinations are up-to-date at least four weeks before starting treatment.
- Screen for infections like hepatitis B and tuberculosis.
- Inebilizumab is not recommended for individuals with active infections or a history of severe reactions to similar treatments.
Pregnancy and Breastfeeding:
- Inebilizumab may harm an unborn baby, so effective contraception should be used during treatment and for six months after the last dose.
- It may not be safe to breastfeed while using inebilizumab; consult your doctor for advice.
Side Effects
Common Side Effects:
- Painful urination
- Joint pain
Serious Side Effects:
- Allergic reactions (hives, difficulty breathing)
- Infections (fever, chills, body aches, sore throat)
- Hepatitis B reactivation
- Serious brain infections (progressive multifocal leukoencephalopathy)
Patients should report any new or worsening symptoms to their healthcare provider immediately.
Conclusion
Inebilizumab, marketed as Uplizna, is an FDA-approved treatment for NMOSD, helping to reduce relapses and manage symptoms in affected individuals. Approved on June 11, 2020, it offers a new option for patients with this debilitating condition.
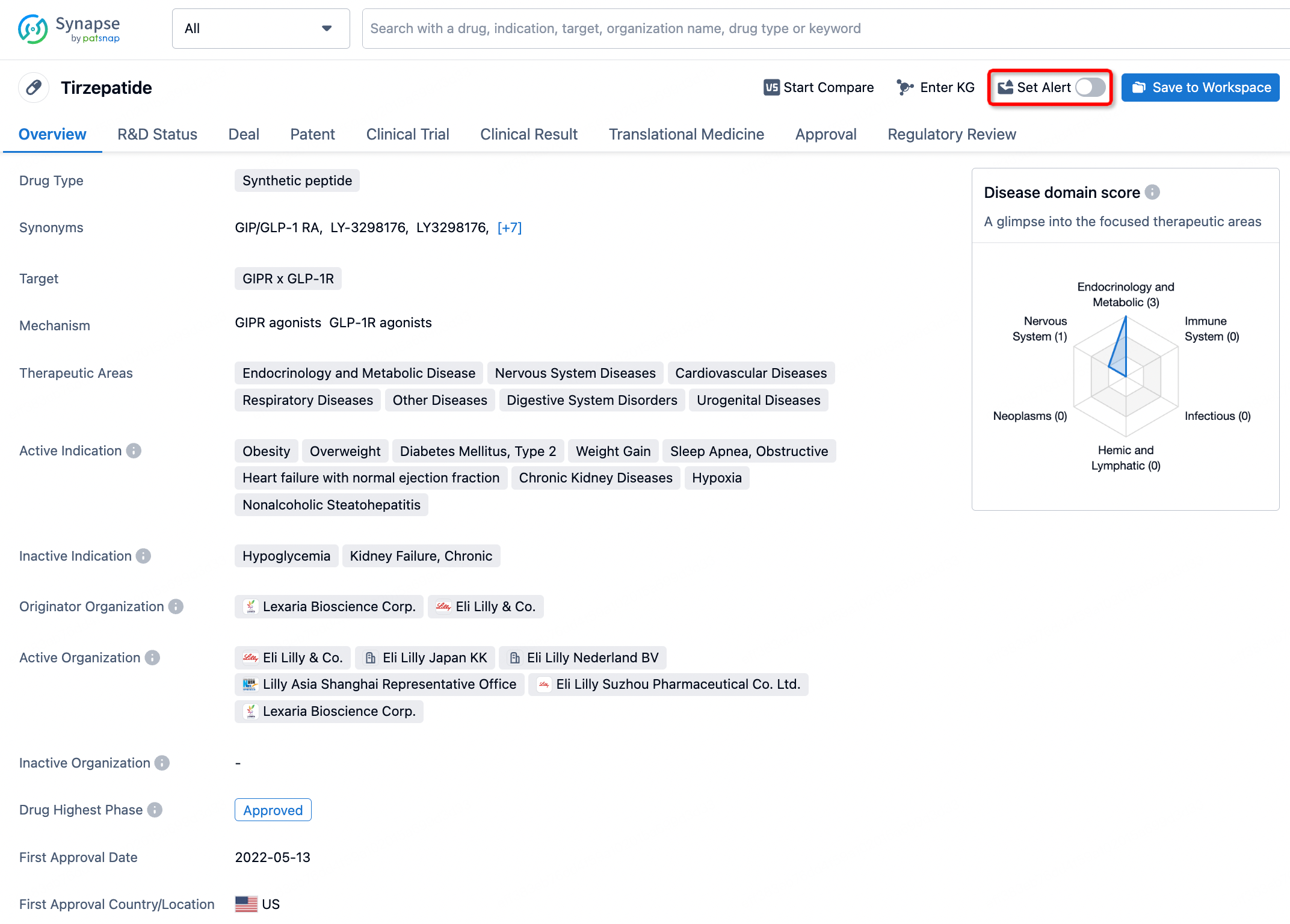
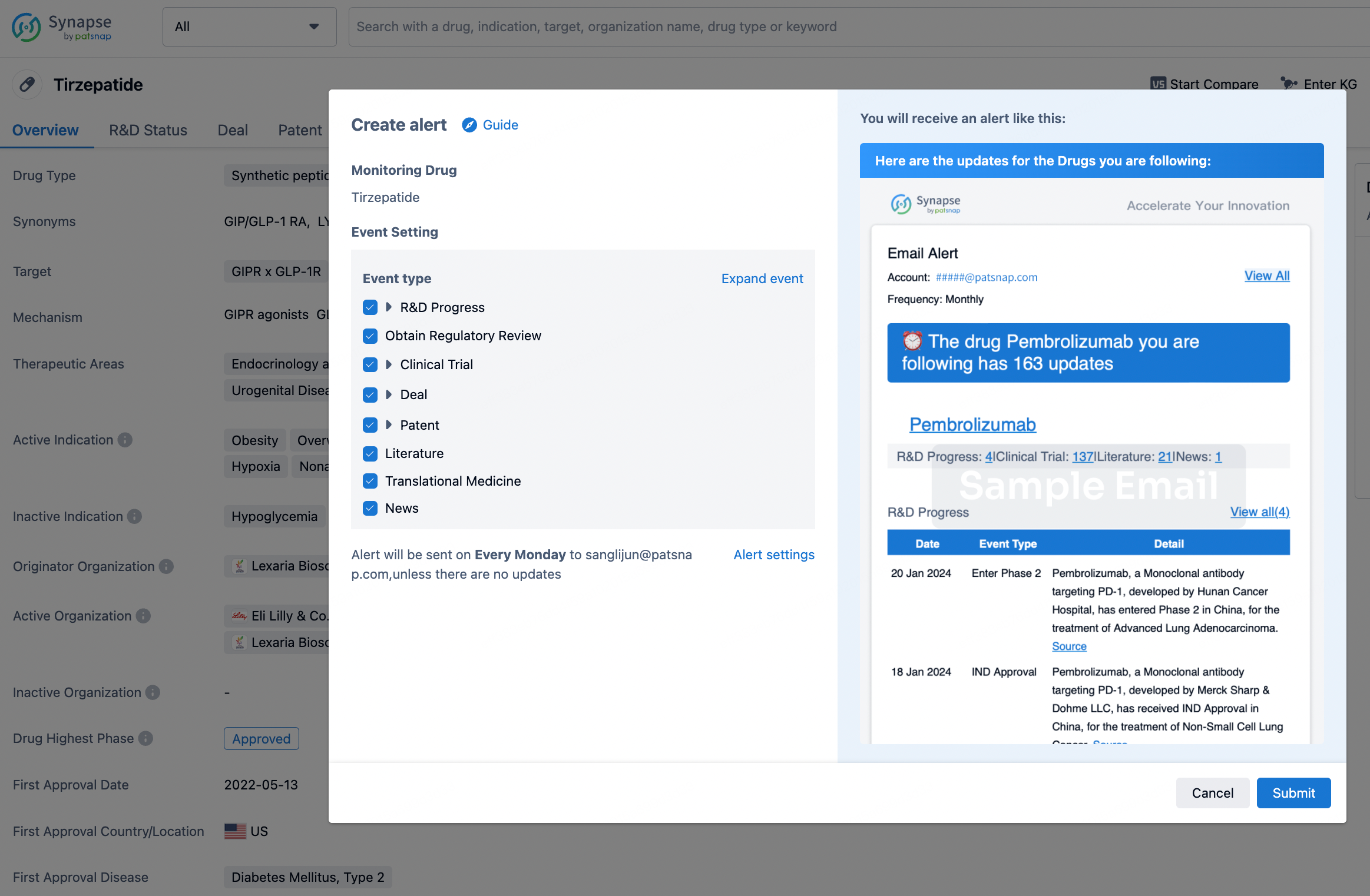
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!