Is Oriahnn approved by the FDA?
Yes, Oriahnn (generic name: elagolix, estradiol, and norethindrone) is FDA approved. The U.S. Food and Drug Administration (FDA) approved Oriahnn on May 29, 2020.
What is Oriahnn?
Oriahnn is a combination of three active ingredients: elagolix, estradiol, and norethindrone. It is used to control heavy menstrual bleeding in adult women with uterine fibroids. It is not intended for use in women who have entered menopause.
How Does Oriahnn Work?
Oriahnn contains:
- Elagolix: A gonadotropin-releasing hormone (GnRH) antagonist that reduces the levels of hormones such as estrogen and progesterone.
- Estradiol: A form of estrogen used to mitigate the side effects of low estrogen levels.
- Norethindrone: A form of progesterone that helps balance the effects of estradiol and reduce the risk of endometrial hyperplasia.
Usage and Administration
Oriahnn is available as oral capsules with a specific dosing regimen:
- Morning capsule: Contains 300 mg elagolix, 1 mg estradiol, and 0.5 mg norethindrone.
- Evening capsule: Contains 300 mg elagolix.
The medication should be taken at the same times each day, with or without food, following the doctor's instructions. Treatment duration should not exceed 24 months due to the risk of bone loss.
Precautions and Considerations
Before using Oriahnn:
- Allergies: Inform your doctor of any allergies to medications or other substances.
- Health Conditions: Oriahnn should not be used if you have uncontrolled high blood pressure, heart disease, liver disease, severe migraines, osteoporosis, or a history of hormone-related cancers, among other conditions.
- Pregnancy and Birth Control: Oriahnn is not a contraceptive and will not prevent pregnancy. Use effective non-hormonal birth control methods during treatment and for at least one week after the last dose.
Warnings:
- Blood Clots and Cardiovascular Risks: Oriahnn may increase the risk of blood clots, stroke, or heart attack, especially in women over 35 who smoke or have other risk factors such as high blood pressure or diabetes.
- Bone Density: Long-term use can cause bone loss, which may be partially irreversible.
Side Effects
Common side effects include:
- Hot flashes
- Fatigue
- Headaches
- Irregular menstrual periods
- Hair loss (may be permanent)
Serious side effects requiring immediate medical attention include:
- Signs of heart attack or stroke
- High blood sugar symptoms
- Mood changes or suicidal thoughts
- Liver or gallbladder problems
Monitoring and Follow-Up
Regular monitoring of blood pressure and bone mineral density is necessary during treatment with Oriahnn. Patients should inform their healthcare providers of their Oriahnn use before undergoing any medical tests or surgeries.
Conclusion
Oriahnn is an FDA-approved medication designed to manage heavy menstrual bleeding associated with uterine fibroids in premenopausal women. Approved on May 29, 2020, it combines elagolix, estradiol, and norethindrone to effectively reduce bleeding while managing hormone levels.
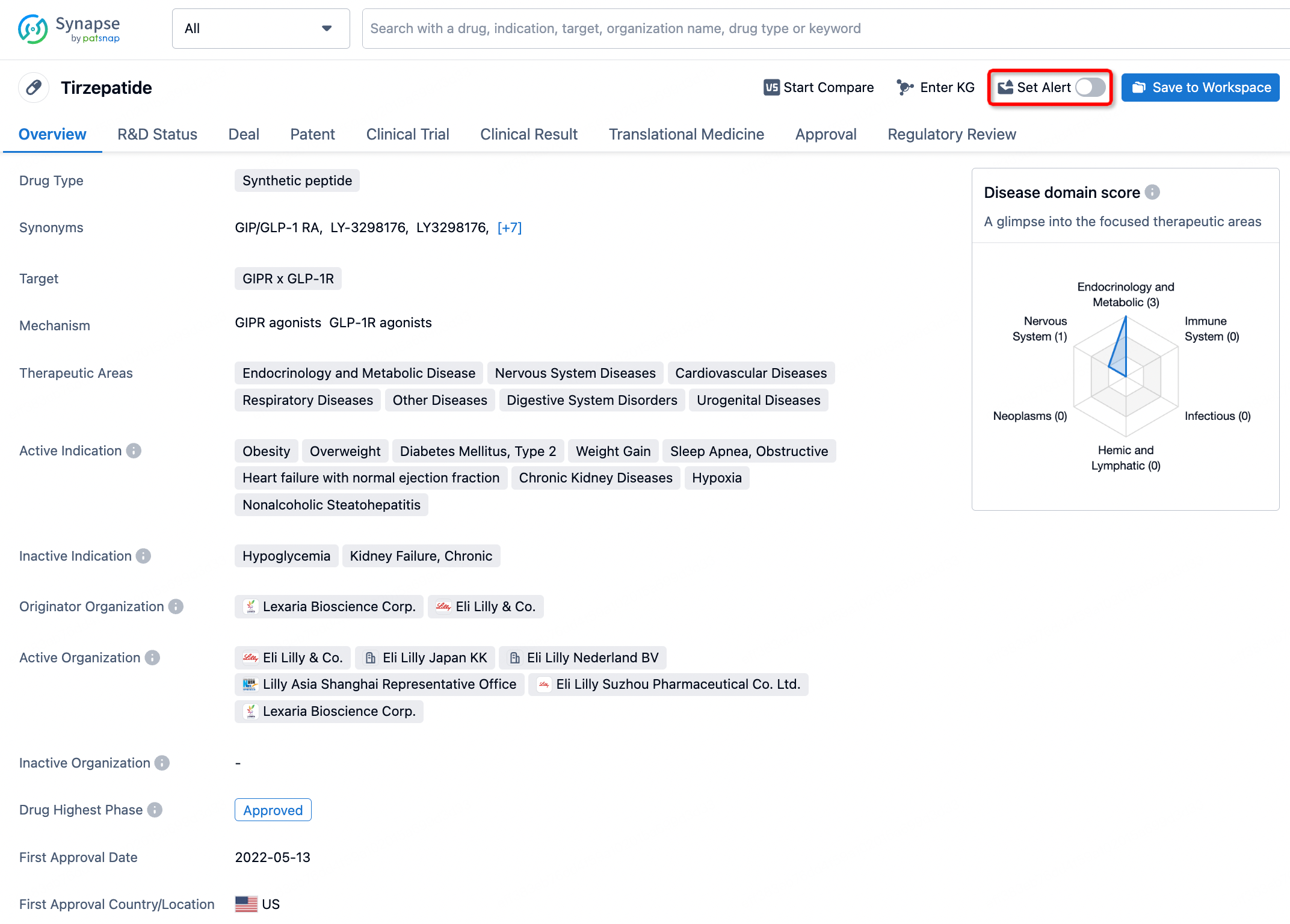
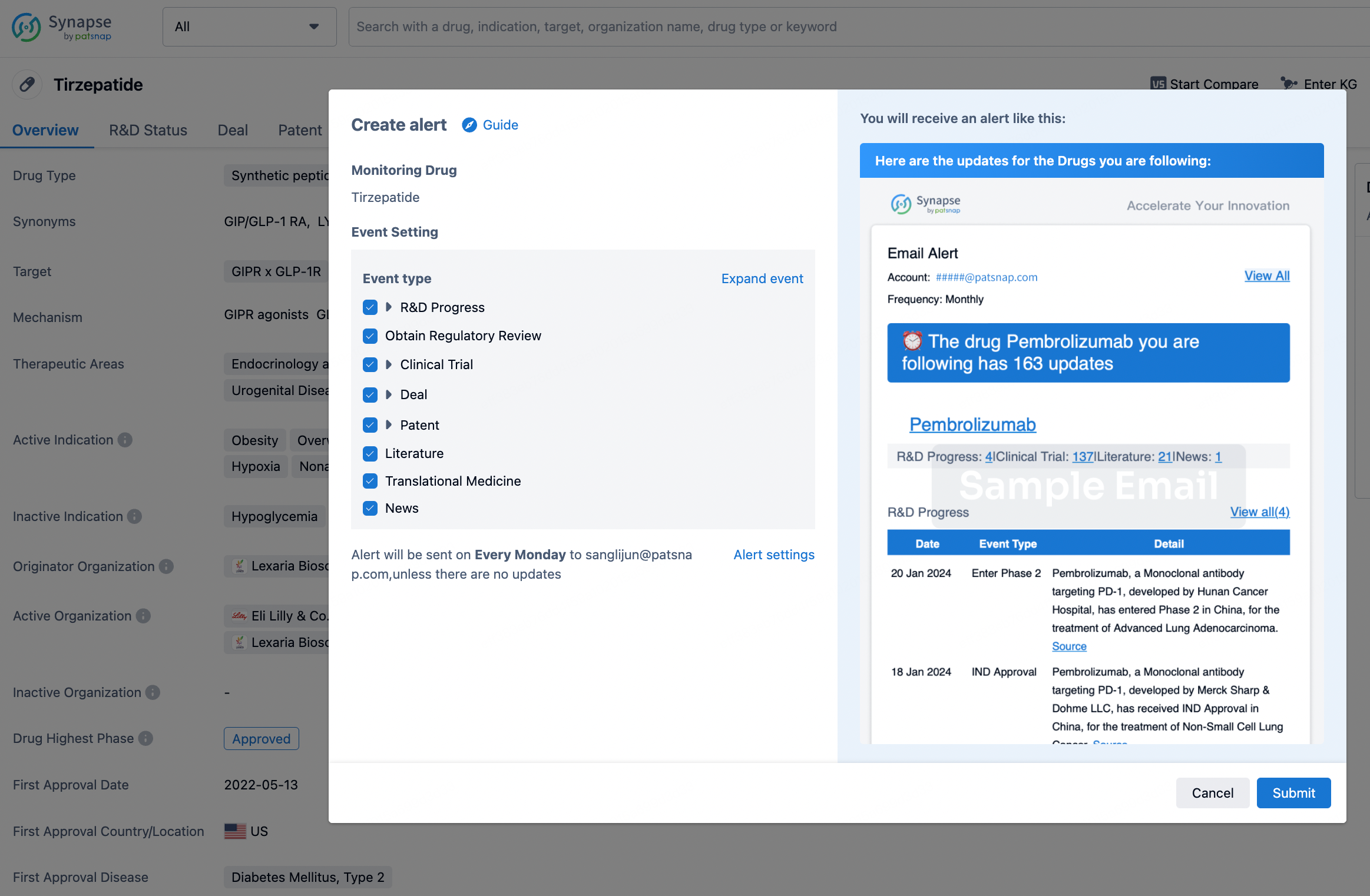
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!