Is Perfluorohexyloctane approved by the FDA?
Perfluorohexyloctane ophthalmic, marketed under the brand name Miebo, is an FDA-approved medication for treating dry eye disease. The approval was granted to this innovative treatment on May 18, 2023, providing a new option for individuals suffering from the discomfort and vision issues associated with dry eye disease.
What is Perfluorohexyloctane Ophthalmic?
Perfluorohexyloctane ophthalmic solution is classified under the drug class of ophthalmic anti-inflammatory agents. It is specifically designed to treat symptoms of dry eye disease by lubricating the eyes, reducing dryness, and alleviating associated discomfort.
Usage and Dosage
- Dosage Form: Ophthalmic solution
- Recommended Dose: Instill one drop 4 times a day into the affected eye(s).
- Administration:
- Wash hands before using the medication.
- Squeeze the bottle in the upright position, then turn it upside down and squeeze again before applying.
- Pull down the lower eyelid to create a small pocket and instill one drop into this pocket.
- Remove contact lenses prior to use and wait at least 30 minutes before reinserting them.
Side Effects
Common side effects of perfluorohexyloctane ophthalmic may include blurred vision. Serious allergic reactions such as hives, difficulty breathing, and swelling of the face, lips, tongue, or throat require immediate medical attention.
Warnings and Precautions
- Use only as directed by your healthcare provider.
- Inform your doctor if you are pregnant or breastfeeding.
- Perfluorohexyloctane ophthalmic is not approved for use by individuals under 18 years old.
Storage
- Store at room temperature in an upright position and tightly closed.
- Do not freeze the medication.
Interactions
While it is unlikely that eye medications will be affected by other drugs, always inform your healthcare provider about all medicines you use, including prescription and over-the-counter drugs, vitamins, and herbal products.
Conclusion
Perfluorohexyloctane ophthalmic (Miebo) is an FDA-approved medication for the treatment of dry eye disease. Approved on May 18, 2023, this solution offers relief from the symptoms of dry eye, such as dryness and discomfort, and provides a new option for those seeking effective treatment. Always use the medication as directed by your healthcare provider and be aware of potential side effects and interactions.
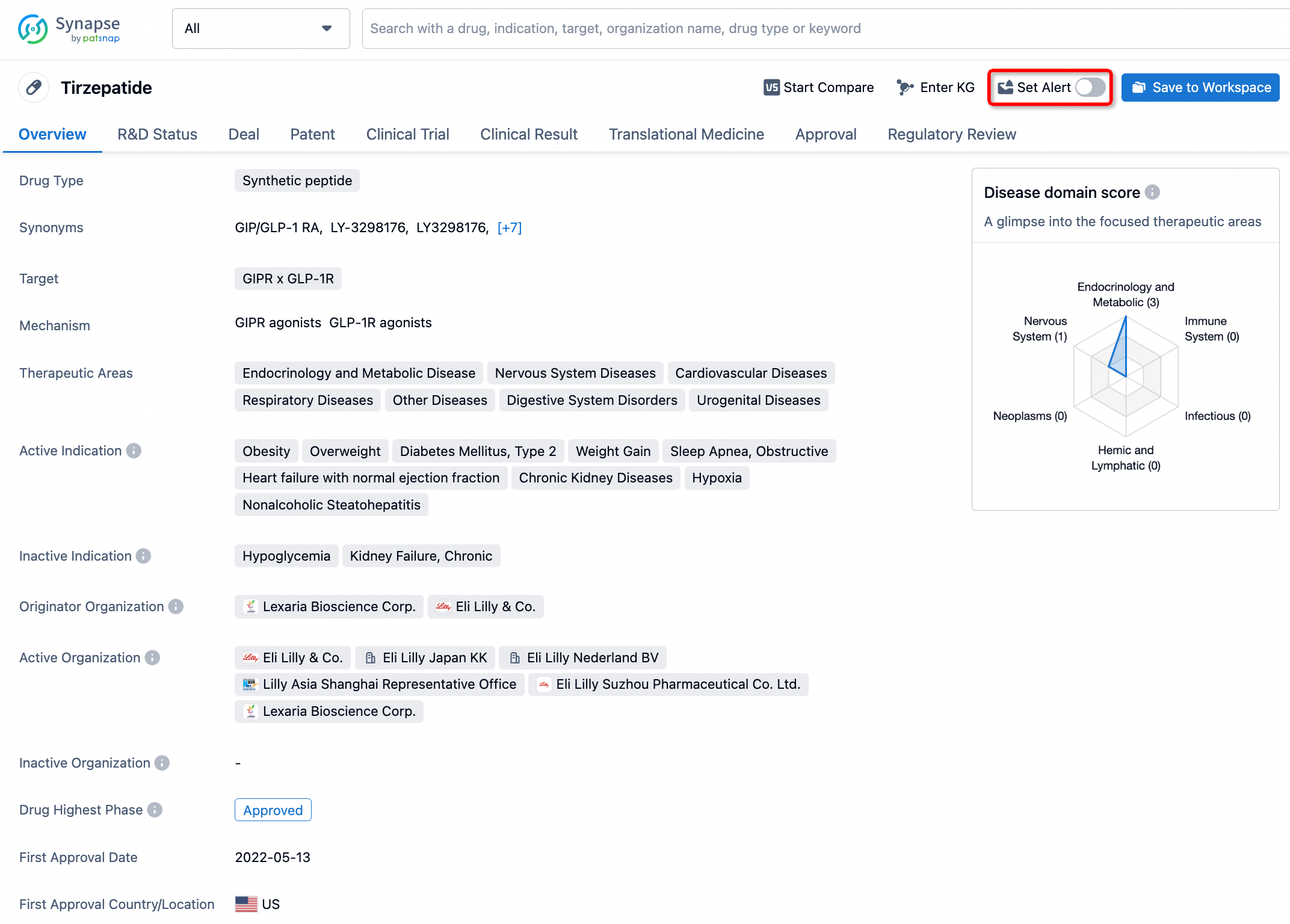
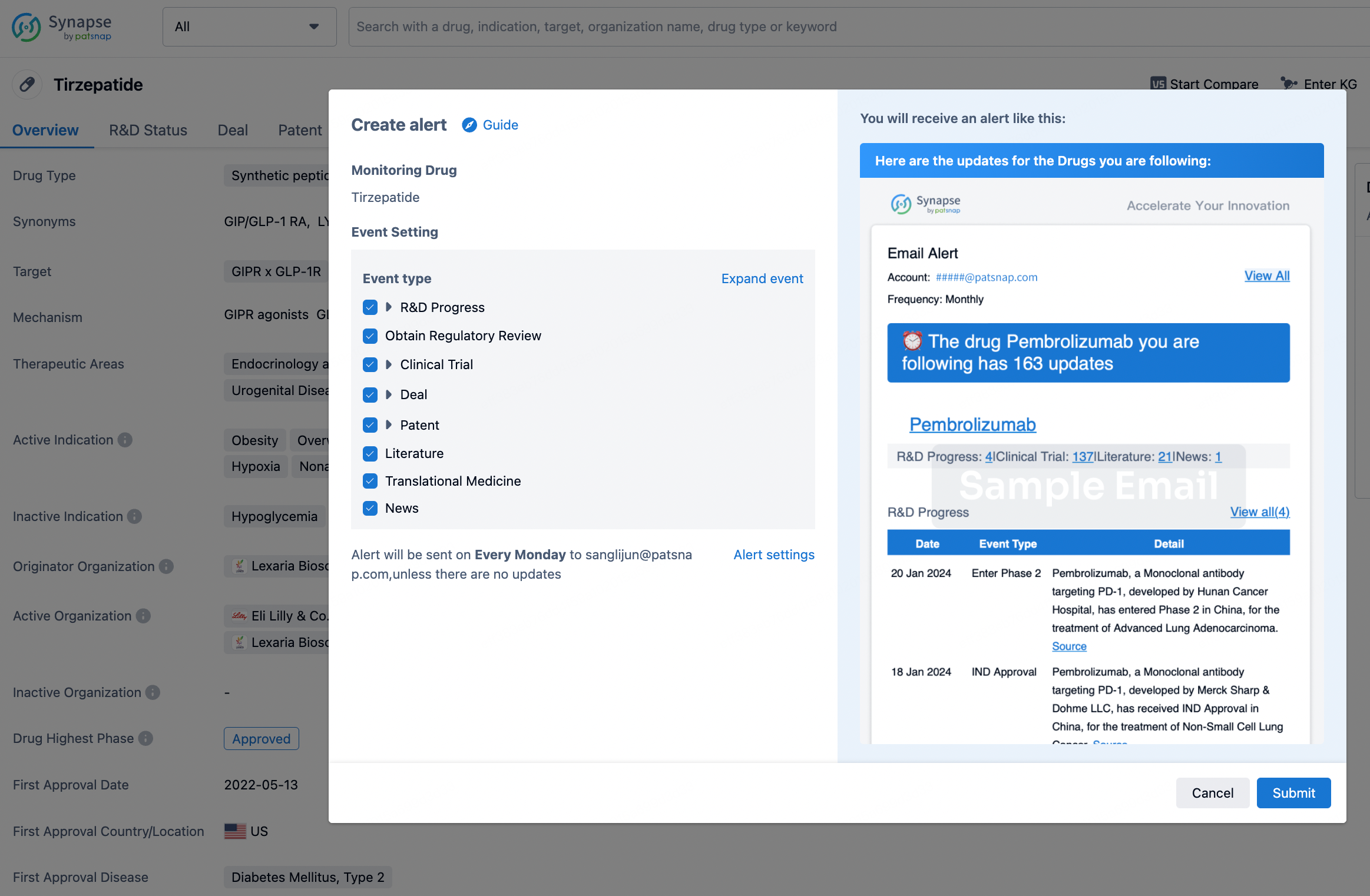
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!