Is Pozelimab approved by the FDA?
Yes, Pozelimab, marketed under the brand name Veopoz, is FDA approved. It received FDA approval for the treatment of complement hyperactivation, angiopathic thrombosis, protein-losing enteropathy (CHAPLE) syndrome in adults and children at least 1 year old.
Uses
Pozelimab is indicated for treating complement hyperactivation, angiopathic thrombosis, protein-losing enteropathy (CHAPLE) syndrome. This condition involves a rare genetic disorder that causes severe gastrointestinal problems and immune system dysfunction.
Mechanism of Action
Pozelimab is a selective immunosuppressant that works by inhibiting specific components of the immune system to reduce the hyperactivation and subsequent damage caused by CHAPLE syndrome. This helps in controlling the symptoms and progression of the disease.
Dosage and Administration
- Administration: Pozelimab is administered by a healthcare provider via intravenous infusion and subcutaneous injection.
- Initial Dose: 30 mg/kg intravenously on Day 1.
- Maintenance Dose: 10 mg/kg subcutaneously once a week starting from Day 8. The maximum dose is 800 mg once a week. The dosage may be increased to 12 mg/kg if there is an insufficient clinical response.
- Monitoring: Patients need to be monitored for at least 30 minutes post-infusion to watch for allergic reactions and other side effects.
Side Effects
Common side effects include:
- Hair loss
- Bone fracture
- Itchy, raised red patches of skin
- Cold symptoms such as stuffy nose, sneezing, sore throat
Serious side effects include:
- Signs of allergic reactions: hives, difficulty breathing, swelling of face, lips, tongue, or throat.
- Infections: fever, chills, sore throat, headache, neck stiffness, increased sensitivity to light, nausea, vomiting, confusion, drowsiness, mouth sores, red or swollen gums, pale skin, easy bruising, unusual bleeding, chest discomfort, wheezing, dry cough, rapid weight loss.
Patients are advised to contact their doctor immediately if they experience any serious side effects. Side effects can also be reported to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Infections: Pozelimab increases the risk of infections, including serious and potentially fatal ones. Patients should be vigilant for symptoms of infections such as flu symptoms, cough, night sweats, headache, neck stiffness, increased sensitivity to light, confusion, or vision problems.
- Vaccination: Patients should be up-to-date with all vaccinations before starting Pozelimab. This includes the meningococcal vaccine, which should be administered at least 2 weeks prior to the first dose.
- Pregnancy and Breastfeeding: Pozelimab may harm an unborn baby. Women should use effective birth control during treatment and for at least 4 months after the last dose. Breastfeeding is not recommended during treatment and for at least 4 months after the last dose.
Additional Information
Pozelimab is only available through certified pharmacies and must be administered under a special program. Patients should carry a Patient Wallet Card that outlines symptoms of meningococcal or other infections to watch for and must keep this card with them at all times.
Summary
Pozelimab (Veopoz) is FDA approved for the treatment of complement hyperactivation, angiopathic thrombosis, protein-losing enteropathy (CHAPLE) syndrome in both adults and children aged 1 year and older. It is administered as an intravenous infusion followed by subcutaneous injections and requires careful monitoring due to potential serious side effects and increased risk of infections. This approval provides a significant treatment option for patients suffering from this rare and severe genetic disorder.
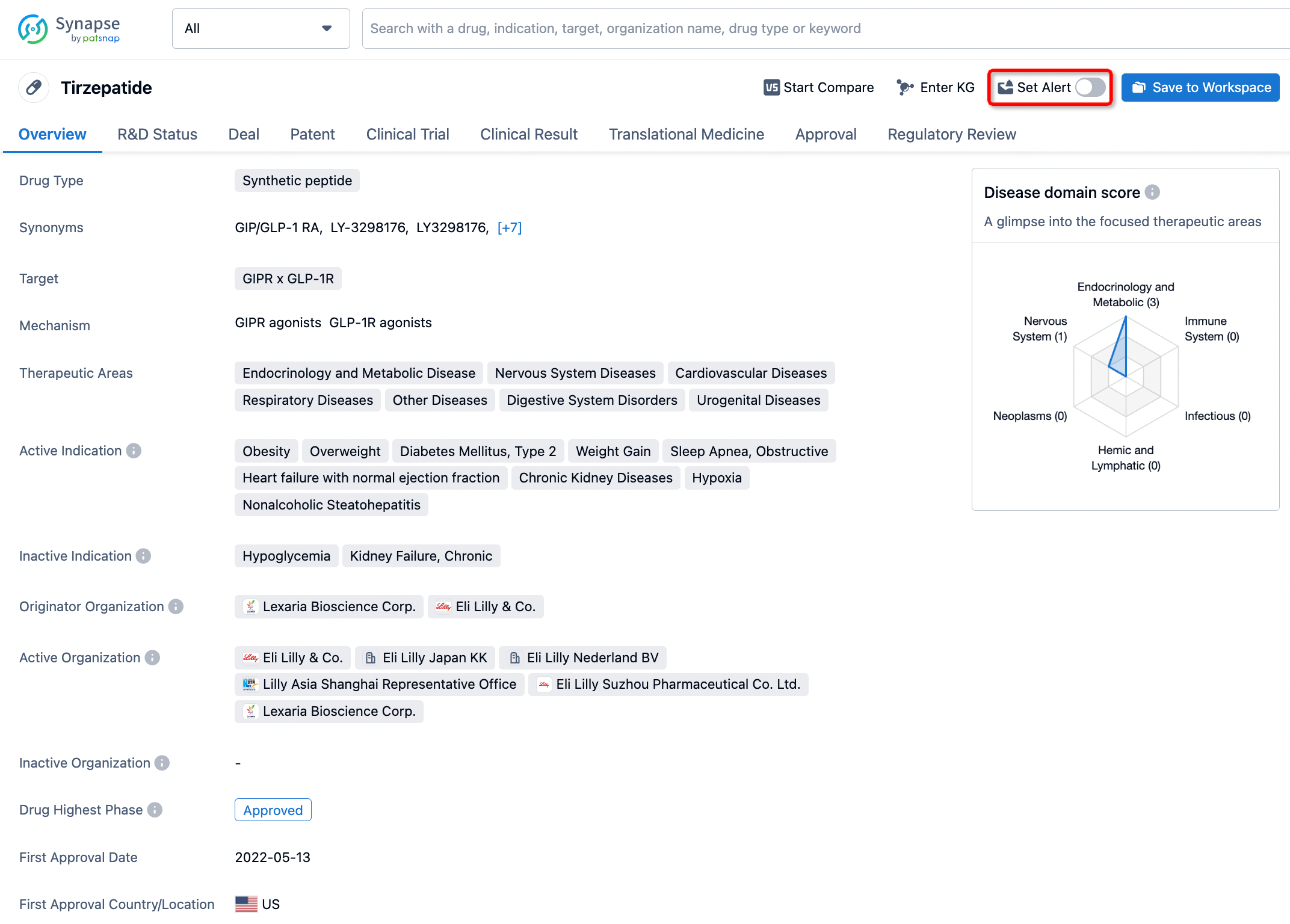
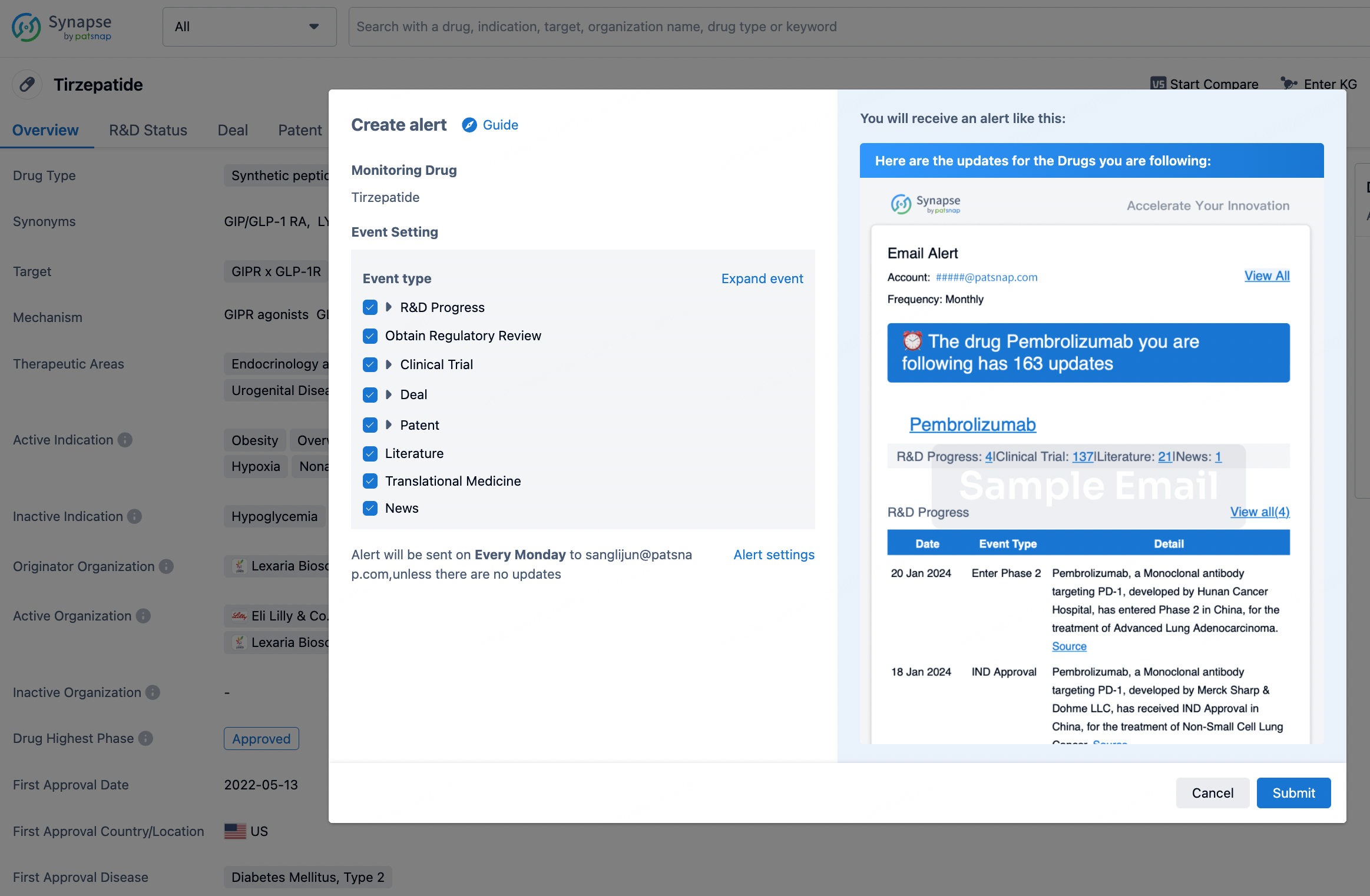
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!