Is Talquetamab approved by the FDA?
Yes, Talquetamab, marketed under the brand name Talvey, is FDA approved. It received approval for the treatment of adults with multiple myeloma who have received at least four prior treatment regimens, and whose cancer has either returned or did not respond to previous treatments.
Uses
Talquetamab is used to treat adults with multiple myeloma, a type of blood cancer, particularly for those who have exhausted at least four prior treatment options. Multiple myeloma is a complex and aggressive cancer, and Talquetamab offers a new therapeutic option for patients with refractory or relapsed forms of this disease.
Mechanism of Action
Talquetamab is part of the miscellaneous antineoplastics drug class, which encompasses a variety of cancer-fighting medications with different mechanisms of action. It is designed to target and kill cancerous cells in multiple myeloma patients, though the specific mechanism of Talquetamab’s action in the immune system and its effects on cancer cells are part of ongoing research and clinical studies.
Dosage and Administration
- Administration: Talquetamab is administered by a healthcare provider as a subcutaneous injection, meaning it is injected under the skin, typically in the stomach area, thigh, or another appropriate site.
- Monitoring: Patients receiving Talquetamab will need to stay in the hospital for 48 hours after certain doses to monitor for serious side effects or allergic reactions. Medical tests are required before and during treatment to ensure safety and efficacy.
Side Effects
Common side effects include:
- Changes in sense of taste
- Difficulty swallowing, dry mouth
- Diarrhea, weight loss
- Muscle or joint pain
- Fatigue
- Nail problems (fingernails or toenails)
- Dry skin and mucous membranes
- Fever, sinus, or throat infection
Serious side effects include:
- Signs of cytokine release syndrome (CRS): fever, chills, trouble breathing, confusion, severe vomiting or diarrhea, irregular heartbeats, feeling light-headed or very tired.
- Increased risk of infections: symptoms include fever, chills, sore throat, mouth sores, red or swollen gums, pain or burning during urination, pale skin, easy bruising, unusual bleeding, chest discomfort, wheezing, dry cough, and rapid weight loss.
- Neurological problems: headache, jerking muscle movements, rigid muscles, restlessness, numbness and tingling, confusion, problems with speech or walking, muscle weakness, hearing loss, and pain sensations.
- Liver problems: symptoms include loss of appetite, stomach pain (upper right side), tiredness, itching, dark urine, clay-colored stools, and jaundice (yellowing of the skin or eyes).
Patients should report any side effects to their doctor and can also report them to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Talquetamab may cause serious side effects such as cytokine release syndrome and neurological issues. Patients should be closely monitored for these conditions.
- Talquetamab can harm an unborn baby. Women should use effective birth control during treatment and for at least 3 months after the last dose.
- Breastfeeding is not recommended while using Talquetamab and for at least 3 months after the last dose.
Additional Information
Talquetamab is available only through a special program due to its potential risks and benefits. Patients must be registered in this program to receive the medication. Before starting treatment, patients are given a Patient Wallet Card with information about serious side effects and symptoms to watch for. They should keep this card with them at all times.
Summary
Talquetamab (Talvey) is FDA approved for the treatment of adults with multiple myeloma who have undergone at least four previous treatment regimens. It offers a new option for those with refractory or relapsed forms of this aggressive cancer. Administered as a subcutaneous injection, Talquetamab requires careful monitoring due to its potential for serious side effects, including cytokine release syndrome, infections, and neurological problems. The approval of Talquetamab provides hope for patients with limited treatment options in their fight against multiple myeloma.
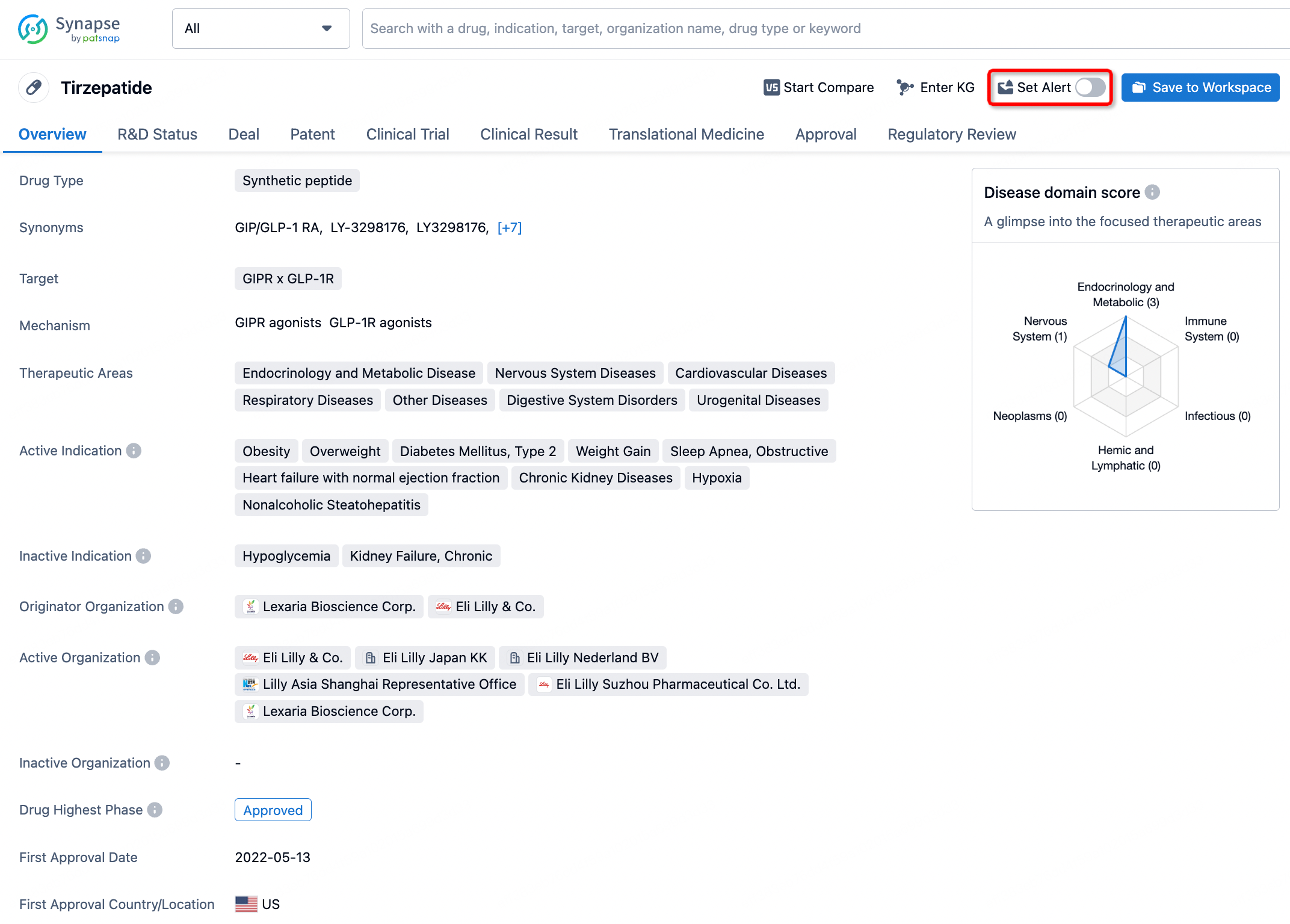
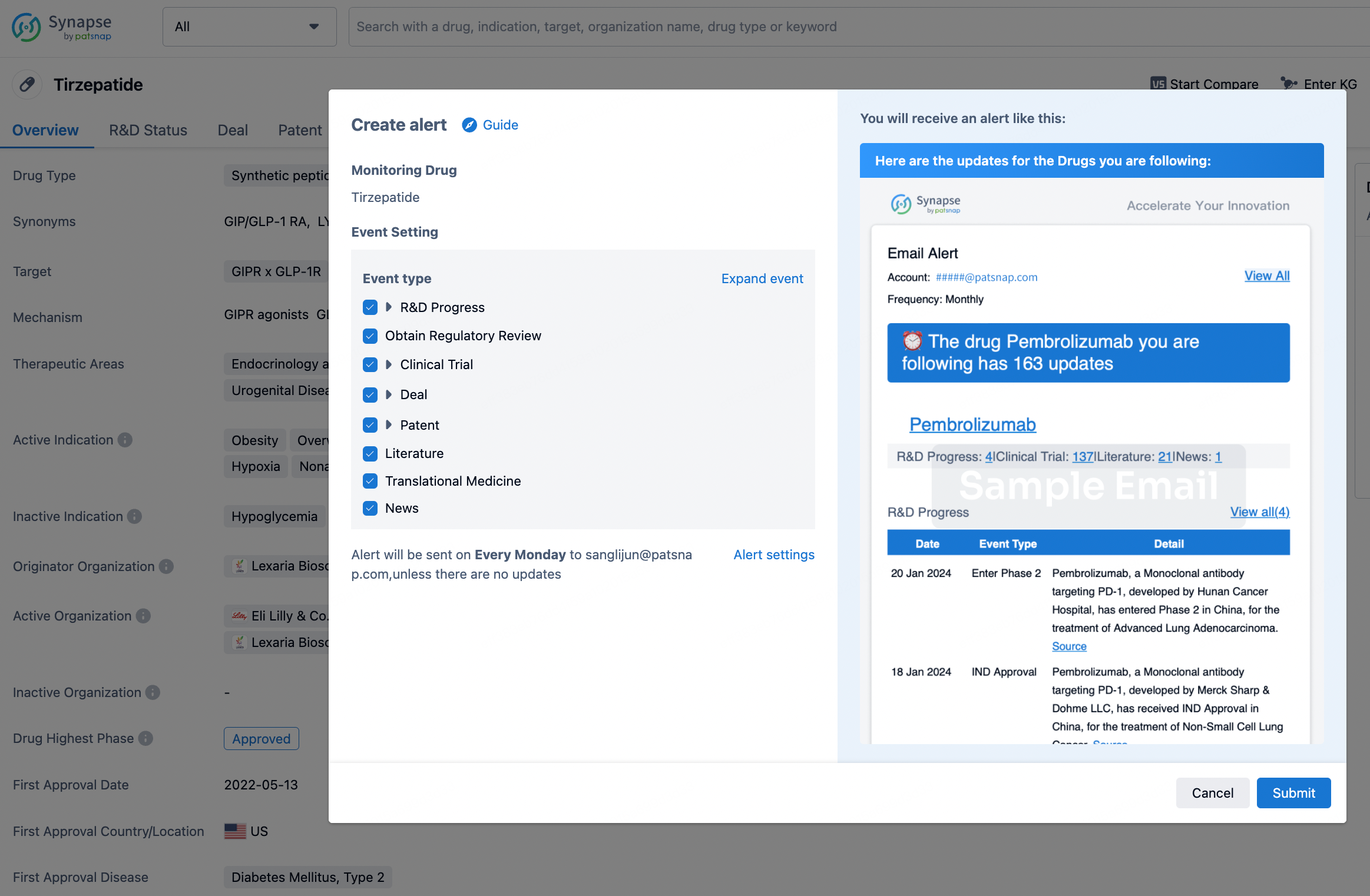
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!