Is Selinexor approved by the FDA?
Yes, selinexor, marketed under the brand name Xpovio, is FDA approved. The U.S. Food and Drug Administration (FDA) granted approval for selinexor on an accelerated basis on July 3, 2019. This approval is based on the medication's ability to meet an unmet medical need in patients with specific types of cancer.
What is Selinexor?
Selinexor is an oral medication used in combination with other drugs to treat multiple myeloma and certain types of diffuse large B-cell lymphoma (DLBCL) in adults. It is a selective inhibitor of nuclear export (SINE) compound, classified under miscellaneous antineoplastics.
Uses of Selinexor
- Multiple Myeloma:
- In combination with bortezomib and dexamethasone for patients who have received at least one prior therapy.
- In combination with dexamethasone for patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
- Diffuse Large B-Cell Lymphoma (DLBCL):
- For the treatment of relapsed or refractory DLBCL, not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.
How is Selinexor Administered?
Selinexor is available in oral tablet forms with various dosing schedules, including once or twice weekly administration, depending on the specific regimen and combination with other medications. Dosages must be carefully followed as prescribed by a healthcare provider.
Common Side Effects
Selinexor can cause a range of side effects, some of which can be serious or fatal. Common side effects include:
- Double vision, blurred vision, sensitivity to light or glare
- Tiredness
- Numbness, tingling, or burning pain in hands or feet
- Anemia, bruising or bleeding
- Increased blood sugar
- Fever, infections, cold or flu symptoms
- Changes in sodium and mineral levels
- Abnormal liver or kidney function tests
- Nausea, vomiting, loss of appetite
- Diarrhea, constipation
- Weight loss
- Shortness of breath
Serious side effects may include blurred vision, severe nausea, loss of appetite, confusion, symptoms of sepsis, signs of infection, and low sodium levels.
Warnings and Precautions
- Infection Risk: Patients may get infections more easily, including serious or fatal infections.
- Low Platelet Counts: Can cause unusual bruising or bleeding.
- Reproductive Health: Selinexor can harm an unborn baby; effective birth control is required for both men and women using this medication.
Drug Interactions
Selinexor can interact with other medications, including prescription and over-the-counter drugs, vitamins, and herbal products. Patients should inform their healthcare provider about all medications they are taking to avoid potential interactions.
Conclusion
Selinexor (Xpovio) received FDA approval on July 3, 2019, for treating multiple myeloma and diffuse large B-cell lymphoma. As with all medications, it is crucial to follow the prescribed dosage and administration instructions and to be aware of potential side effects and interactions with other drugs.
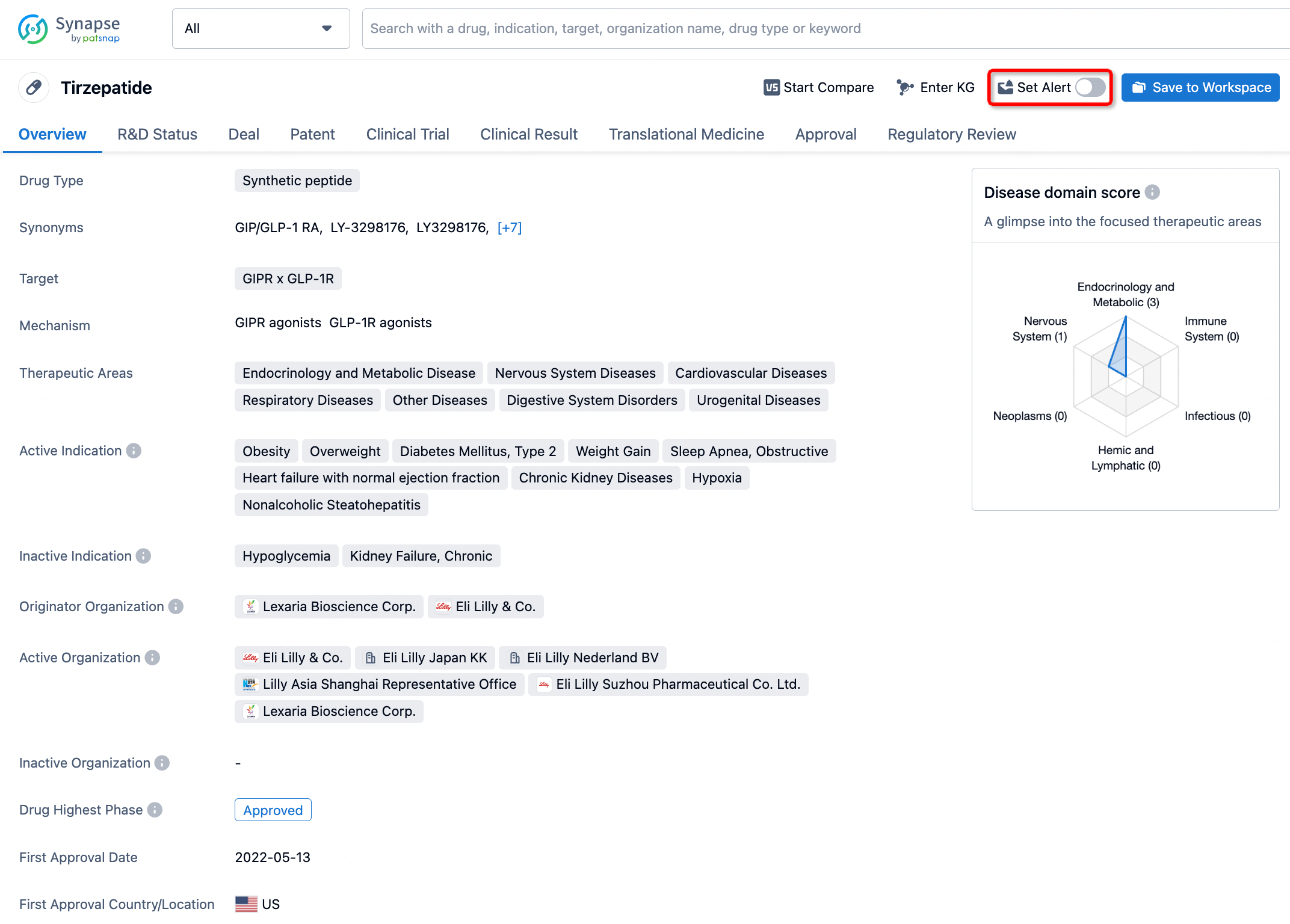
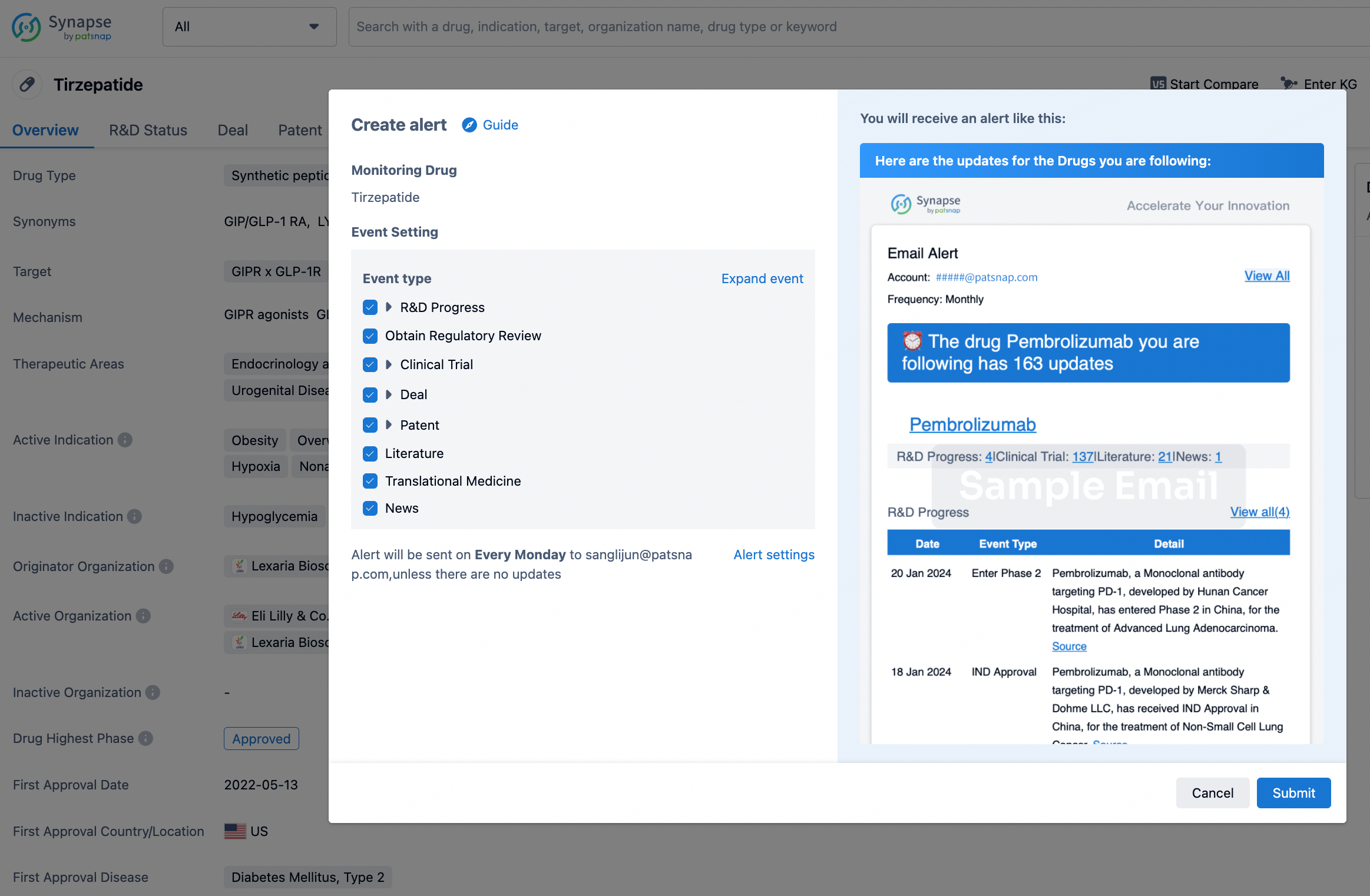
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!