Is Zuranolone approved by the FDA?
Zuranolone, marketed under the brand name Zurzuvae, is approved by the FDA for the treatment of postpartum depression (PPD) in adults. The FDA granted this approval on August 4, 2023, to Sage Therapeutics, Inc. The approval was based on clinical trials, including the LANDSCAPE and NEST studies, which demonstrated its efficacy in treating PPD.
Uses
Zuranolone is used for the treatment of postpartum depression (PPD). PPD is a serious condition that can occur after childbirth, characterized by symptoms such as intense anxiety, sadness, shame, guilt, problems sleeping, stress, panic attacks, and suicidal thoughts or attempts. It can interfere with a mother’s ability to care for and bond with her baby, potentially impacting the child’s development.
Mechanism of Action
Zuranolone is a neuroactive steroid that acts as a GABA-A receptor positive allosteric modulator (PAM). It is believed to rebalance brain networks responsible for mood, arousal, behavior, and cognition, addressing imbalances in the neurotransmitter GABA (Gamma-aminobutyric acid). This rapid-acting medication begins to improve depression symptoms within three days, compared to the weeks or months often required for other treatments.
Dosage and Administration
- Recommended dosage: 50 mg taken once daily in the evening with fatty food.
- Duration: 14 days.
- Alternative dosage: For patients unable to tolerate the 50 mg dose, it may be reduced to 40 mg once daily.
Effectiveness
In clinical trials, Zuranolone demonstrated significant efficacy in reducing symptoms of postpartum depression. A key study (Study 1, NCT04442503) showed that women taking Zuranolone 50 mg daily for two weeks experienced a statistically significant reduction in depressive symptoms by day 15, measured using the Hamilton Rating Scale for Depression (HAMD-17). The reduction in the HAMD-17 score was -15.6 for Zuranolone compared to -11.6 for placebo, a difference of -4.0. Improvements in symptoms were observed as early as day 3 and lasted through day 42.
Side Effects
Common side effects of Zuranolone include:
- Sleepiness or drowsiness
- Tiredness
- Dizziness
- Common cold
- Diarrhea
- Urinary tract infection
Serious side effects include an increased risk of suicidal thoughts or actions, especially in individuals 24 years of age and younger. It is crucial to monitor for any sudden changes in mood, behavior, thoughts, or feelings and to seek medical advice if these occur. Other serious side effects can involve decreased alertness and increased risk of falls.
Warnings and Precautions
- Driving and operating machinery: Zuranolone can decrease awareness and alertness, affecting the ability to drive safely or operate machinery. It is advised not to engage in these activities until at least 12 hours after taking each dose.
- Alcohol and CNS depressants: Avoid alcohol and other CNS depressants as they can worsen side effects such as sleepiness and dizziness.
Pregnancy and Breastfeeding
- Pregnancy: Zuranolone may harm an unborn baby. Women who can become pregnant should use effective contraception while taking this medication and for one week after the last dose. If pregnancy occurs during treatment, the patient should enroll in the National Pregnancy Registry for Antidepressants.
- Breastfeeding: Zuranolone passes into breast milk, and it is not known if it can harm the baby. Patients should discuss the risks and benefits with their healthcare provider.
Drug Interactions
Zuranolone can interact with various medications, including antidepressants, opioids, and CNS depressants. Patients should inform their healthcare provider about all medications they are taking, including prescription, over-the-counter, vitamins, and herbal products.
Summary
Zuranolone, approved by the FDA on August 4, 2023, as the first oral medication for postpartum depression, offers a rapid-acting treatment option that can significantly reduce depressive symptoms within a few days. Its approval marks a significant advancement in the treatment of PPD, providing a convenient and effective alternative to existing therapies.
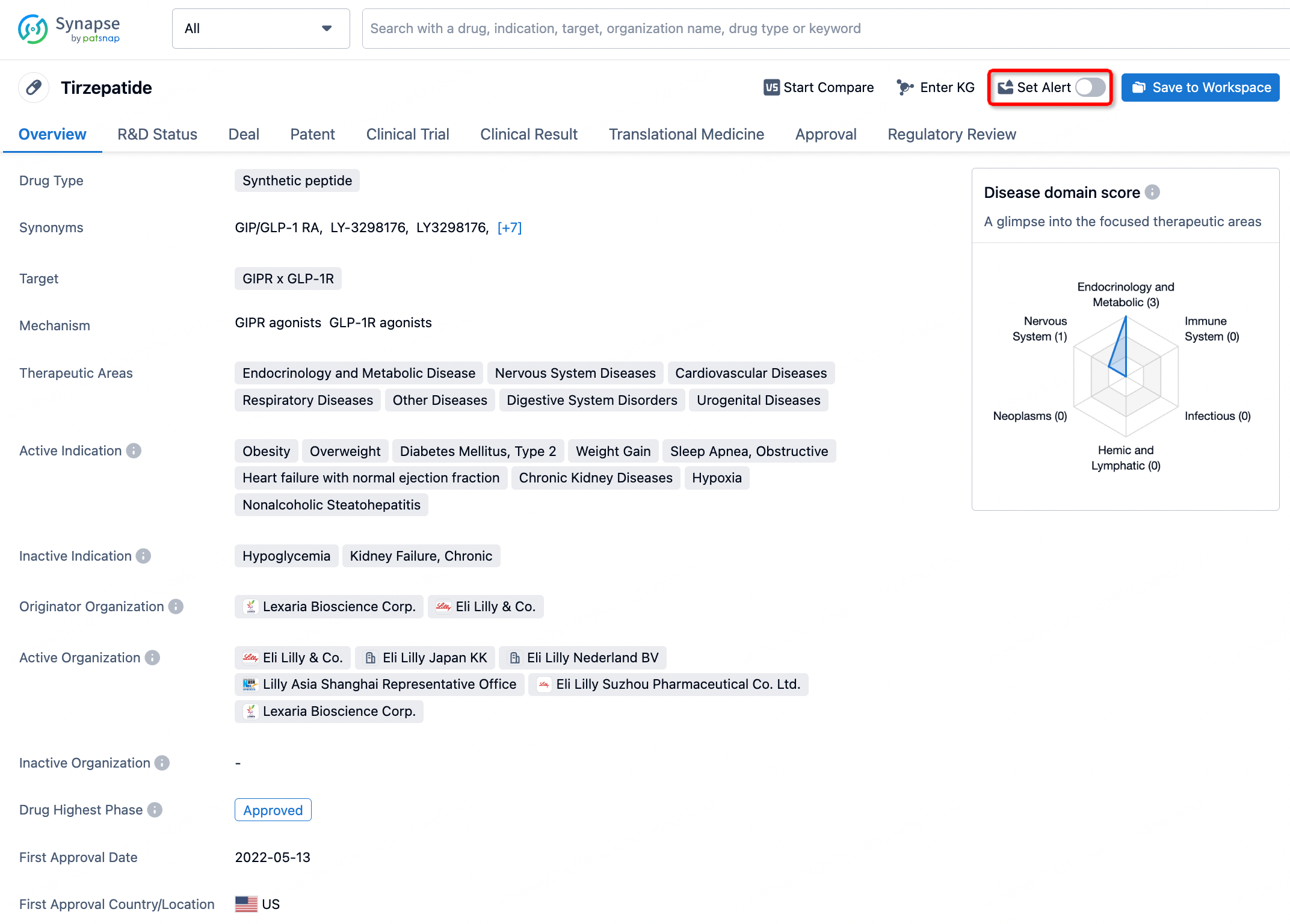
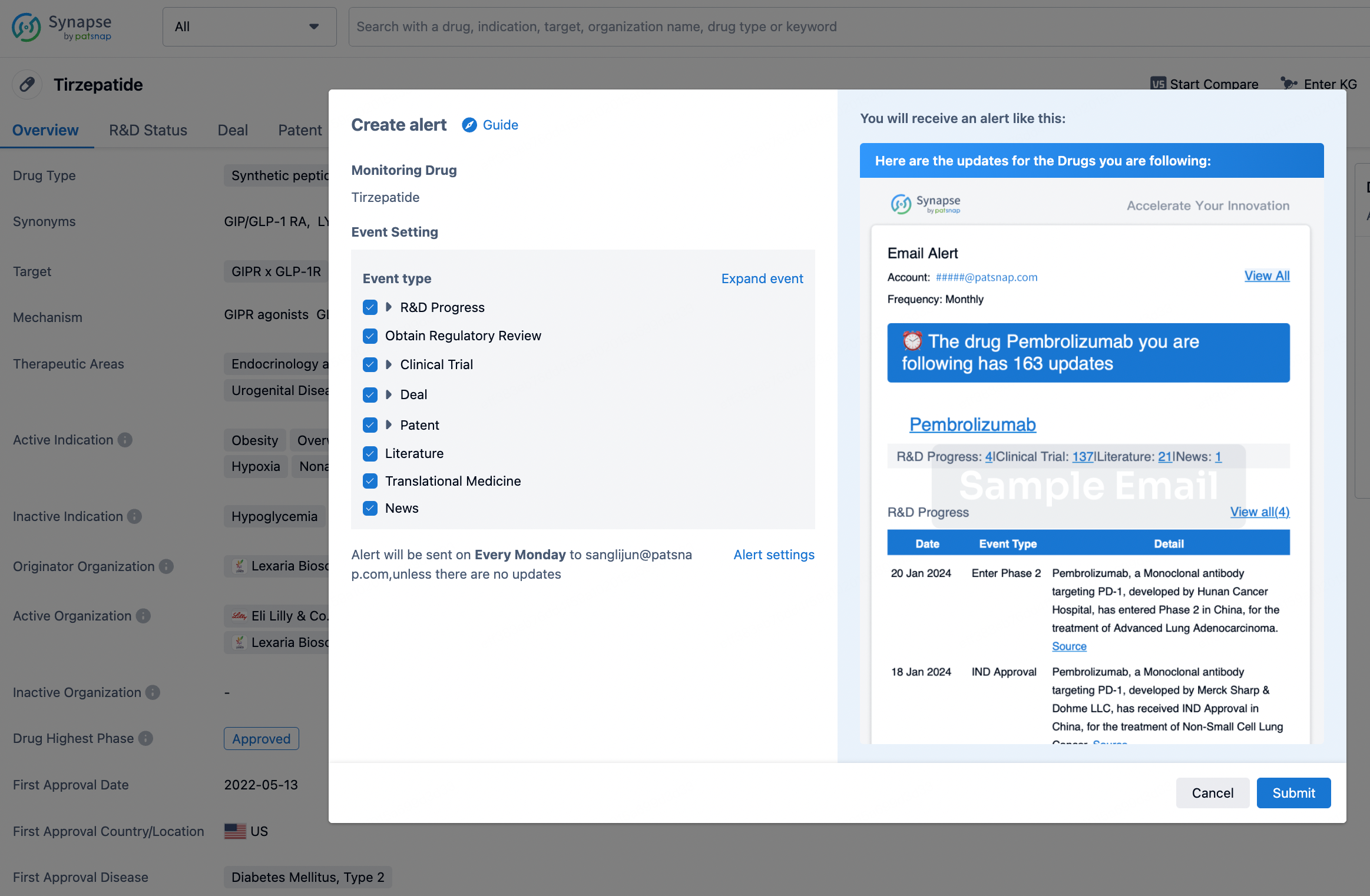
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!