Is Teprotumumab approved by the FDA?
Yes, Teprotumumab, marketed under the brand name Tepezza, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Teprotumumab on January 21, 2020, for the treatment of Thyroid Eye Disease (TED), also known as Graves' Eye Disease.
What is Teprotumumab?
Teprotumumab is a monoclonal antibody and a growth hormone receptor blocker used primarily to treat Thyroid Eye Disease (TED). TED is an autoimmune disorder where the immune system attacks the tissues around the eyes, leading to symptoms such as eye redness, bulging eyes, vision problems, dry or watery eyes, and difficulty closing the eyes.
How is Teprotumumab Administered?
Teprotumumab is administered as an intravenous infusion. The treatment regimen includes an initial dose followed by maintenance doses:
- Initial Dose: 10 mg/kg intravenously.
- Maintenance Dose: 20 mg/kg intravenously every 3 weeks for a total of 8 doses.
Each infusion is given slowly over 60 to 90 minutes by a healthcare professional.
Side Effects of Teprotumumab
Teprotumumab can cause various side effects, some of which may be serious. Common side effects include:
- Muscle spasms
- Nausea and diarrhea
- Headache and tiredness
- High blood sugar
- Hair loss
- Hearing problems
- Dry skin
- Altered sense of taste
Serious side effects requiring immediate medical attention include:
- Severe diarrhea, stomach cramps, and bowel issues
- High blood sugar with symptoms such as increased thirst, urination, and dry mouth
- Signs of allergic reactions like hives, difficulty breathing, and swelling of the face
Precautions and Warnings
Before starting treatment with Teprotumumab, patients should inform their doctor if they have:
- Inflammatory bowel disease
- Diabetes or hyperglycemia
Teprotumumab may harm an unborn baby, so effective birth control is advised during treatment and for at least 6 months after the last dose. Additionally, breastfeeding is not recommended while using this medication.
Usage and Storage
Teprotumumab should be used exactly as prescribed by a healthcare provider. The drug is stored at room temperature, away from moisture and heat, and is handled by healthcare professionals to ensure proper administration.
Conclusion
Teprotumumab (Tepezza) is an FDA-approved medication for the treatment of Thyroid Eye Disease, offering a targeted therapy option for managing this autoimmune condition. Approved on January 21, 2020, Teprotumumab provides relief from the symptoms associated with TED, improving the quality of life for affected individuals. Patients should consult their healthcare provider to determine if Teprotumumab is appropriate for their condition and to understand the potential side effects and necessary precautions.
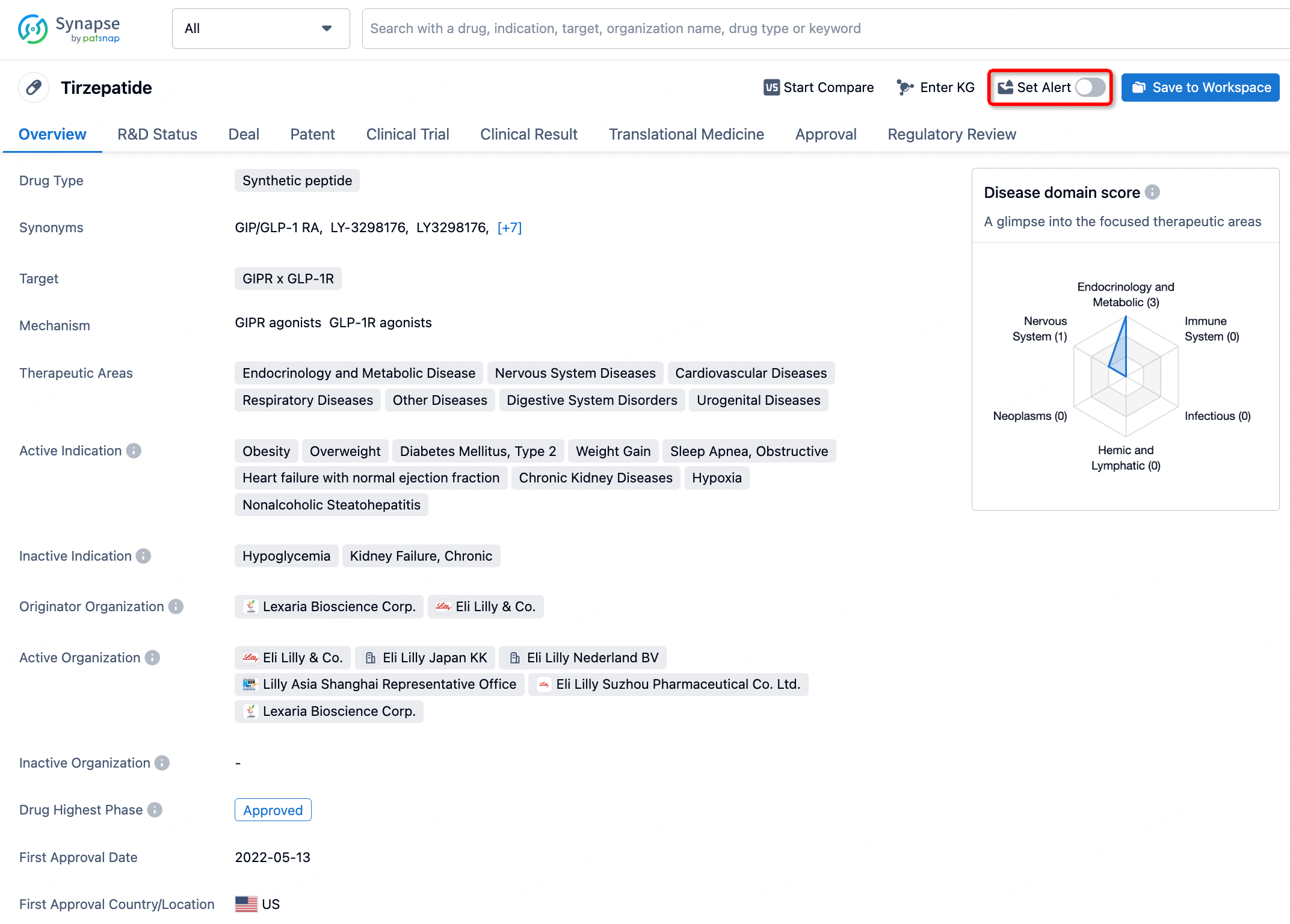
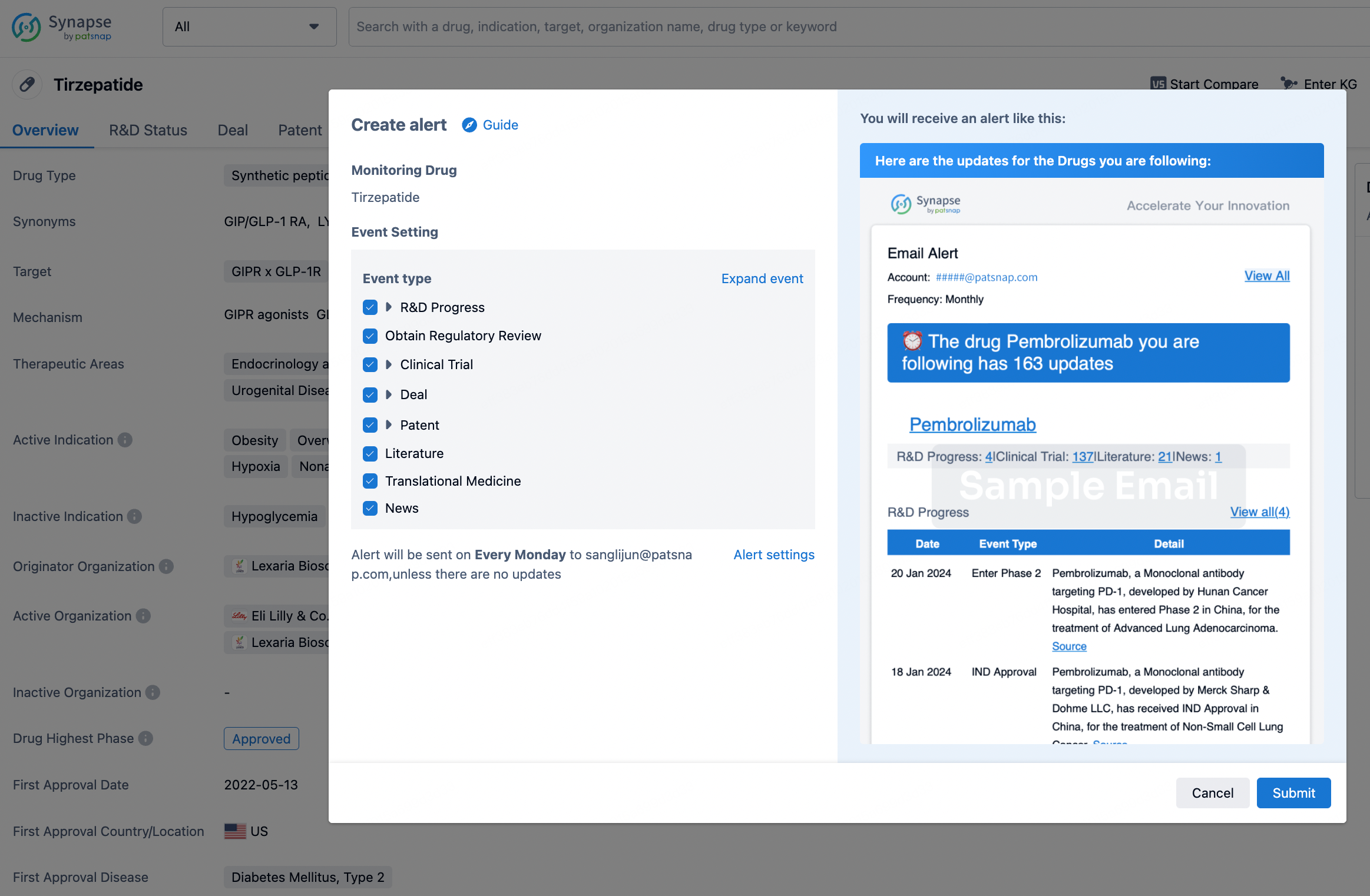
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!