Is Xywav approved by the FDA?
Yes, Xywav is FDA approved. It was first approved on July 21, 2020, for the treatment of cataplexy and excessive daytime sleepiness in patients aged 7 years and older with narcolepsy. Additionally, on August 12, 2021, the FDA expanded its approval to include the treatment of idiopathic hypersomnia (IH) in adults.
What is Xywav?
Xywav is an oral solution that contains a combination of calcium oxybate, magnesium oxybate, potassium oxybate, and sodium oxybate. It is used primarily to treat cataplexy (sudden loss of muscle strength) and reduce excessive daytime sleepiness caused by narcolepsy. Xywav is also used in adults to treat idiopathic hypersomnia (IH), a chronic sleep disorder that causes significant daytime sleepiness even after a good night's sleep.
Key Features and Usage
Xywav is a central nervous system depressant and is known for being a low-sodium alternative to Xyrem. It contains gamma-hydroxybutyrate (GHB), a substance also known for its potential for abuse, which is why Xywav is distributed only through a special restricted distribution program called XYREM REMS. Patients must be registered in this program and understand the risks and benefits of the medication.
FDA-Approved Uses
Xywav is FDA-approved for:
- The treatment of cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy.
- The treatment of idiopathic hypersomnia in adults.
Dosage and Administration
Xywav is typically taken as either a single nightly dose or divided into two doses:
- The first dose is taken at bedtime.
- The second dose is taken 2.5 to 4 hours later.
Both doses must be prepared at the same time, and it is recommended to wait at least 2 hours after eating before taking Xywav. It works quickly, often inducing sleep within 5 to 15 minutes.
Warnings and Precautions
Xywav can cause severe drowsiness and slow or stop breathing. Due to these risks, patients should avoid activities that require full alertness, such as driving or operating heavy machinery, for at least 6 hours after taking Xywav. It is crucial to avoid alcohol and other CNS depressants while taking this medication.
Xywav may be habit-forming, and misuse can lead to addiction, overdose, or death. It is important to keep this medication in a secure place to prevent unauthorized access.
Side Effects
Common side effects include:
- Sleepwalking, talking, or eating in sleep
- Drowsiness, dizziness, headache
- Nausea, vomiting, dry mouth
- Weight loss, bedwetting (in children)
Severe side effects can include:
- Weak or shallow breathing
- Severe drowsiness, confusion, or hallucinations
- Depression, anxiety, or suicidal thoughts
Conclusion
Xywav is an FDA-approved medication for the treatment of narcolepsy-related cataplexy and excessive daytime sleepiness, as well as idiopathic hypersomnia in adults. Due to its potential for abuse and serious side effects, it is available only through a restricted distribution program. Patients using Xywav must adhere to strict guidelines to ensure its safe and effective use.
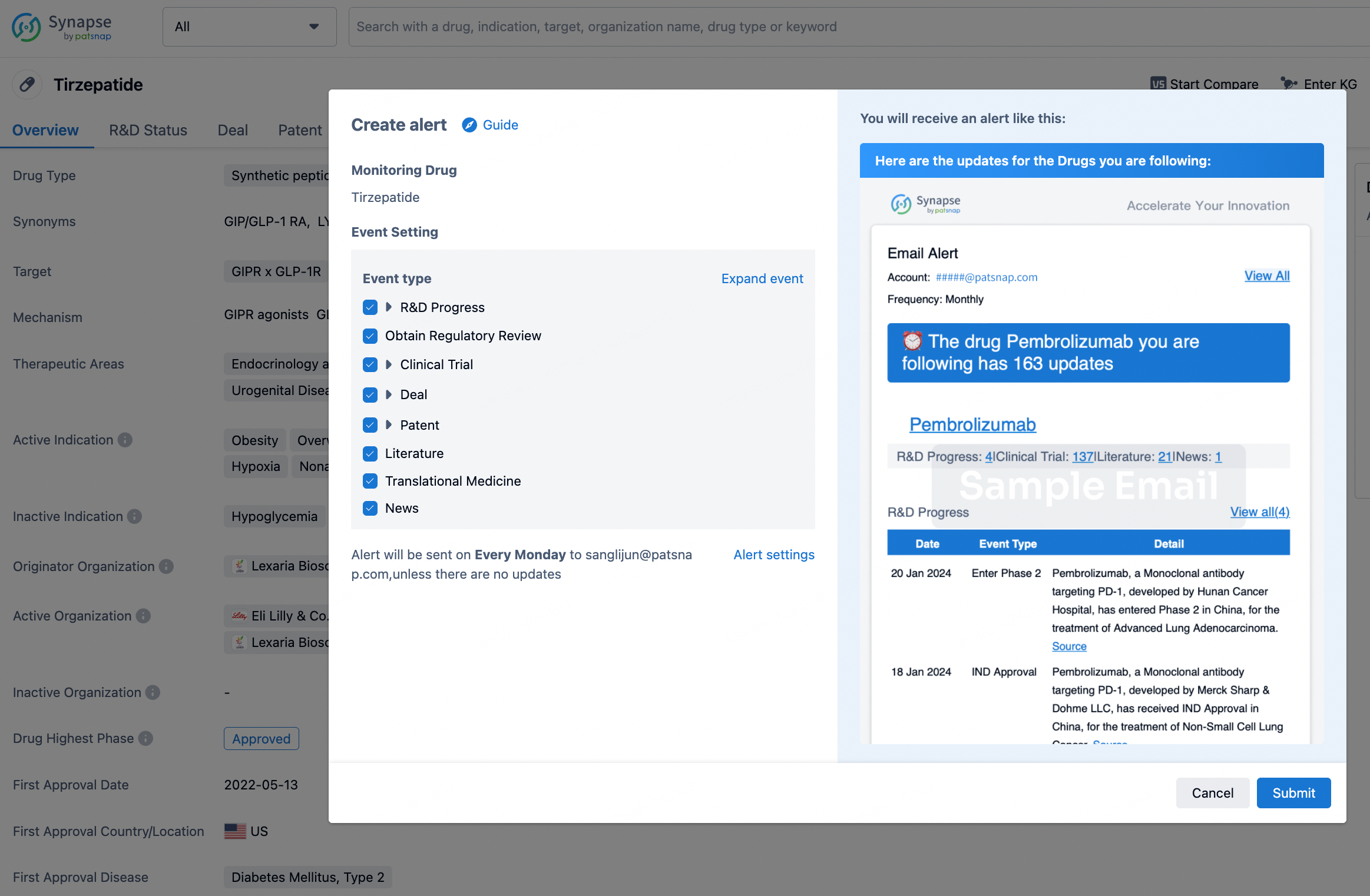
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!