J&J Seeks FDA Approval for TREMFYA® in Treating Adult Ulcerative Colitis
Johnson & Johnson has officially filed for a supplemental Biologics License Application with the FDA, the United States' health authority, to get the green light for their drug, TREMFYA® (guselkumab). This application was made with the intention of using the medication to manage the symptoms of moderately to severely active ulcerative colitis in adult patients.
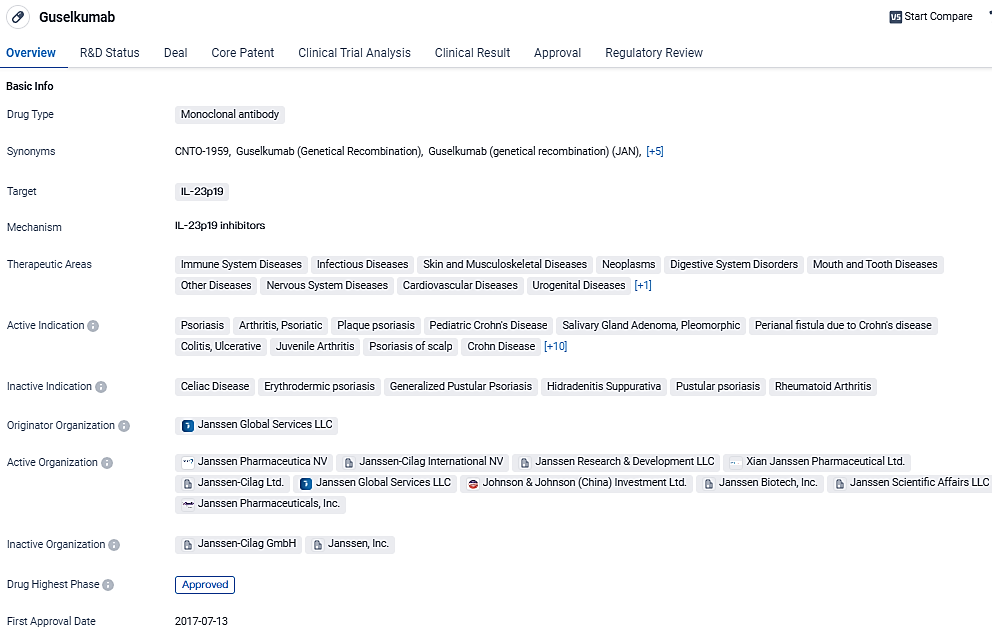
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The application submitted is grounded on findings from the pivotal Phase 3 QUASAR trial, which assessed the effectiveness and safety of TREMFYA® for treating patients with moderate to severe UC, who did not respond adequately to standard treatments, previous biologic therapies, or JAK inhibitors.
Research outcomes revealed significant and substantial enhancements in patient conditions, self-reported health states such as weariness, and indicators of UC severity, achieving rigorous targets like endoscopic and histological remission. TREMFYA®'s safety findings were in line with its established safety profile for previously authorized uses.
"Even with progress in treatment methods, numerous individuals diagnosed with ulcerative colitis still do not achieve satisfactory results from or cannot tolerate current treatments," commented David Lee, MD, PhD, the leader of the Global Immunology Therapeutic Area.
"TREMFYA holds promise as an innovative therapeutic option for these patients. Our team is eager to collaborate with the FDA on the assessment of this submission and we continue to prioritize the development of novel treatments for individuals suffering from chronic autoimmune diseases like ulcerative colitis, who deal with continuous and distressing symptoms," Lee further stated.
TREMFYA® functions as an IL-23 inhibitor, disrupting the activity of IL-23 and attaching to the CD64 receptor found on cells that secrete IL-23. IL-23 is a cytokine released by activated monocytes/macrophages and dendritic cells and it plays a critical role in the pathology of immune-driven conditions, including UC. The first approval of TREMFYA® in the United States came in July 2017 for curing moderate-to-severe plaque psoriasis in adults, with a subsequent approval in July 2020 for treating adults with active psoriatic arthritis.
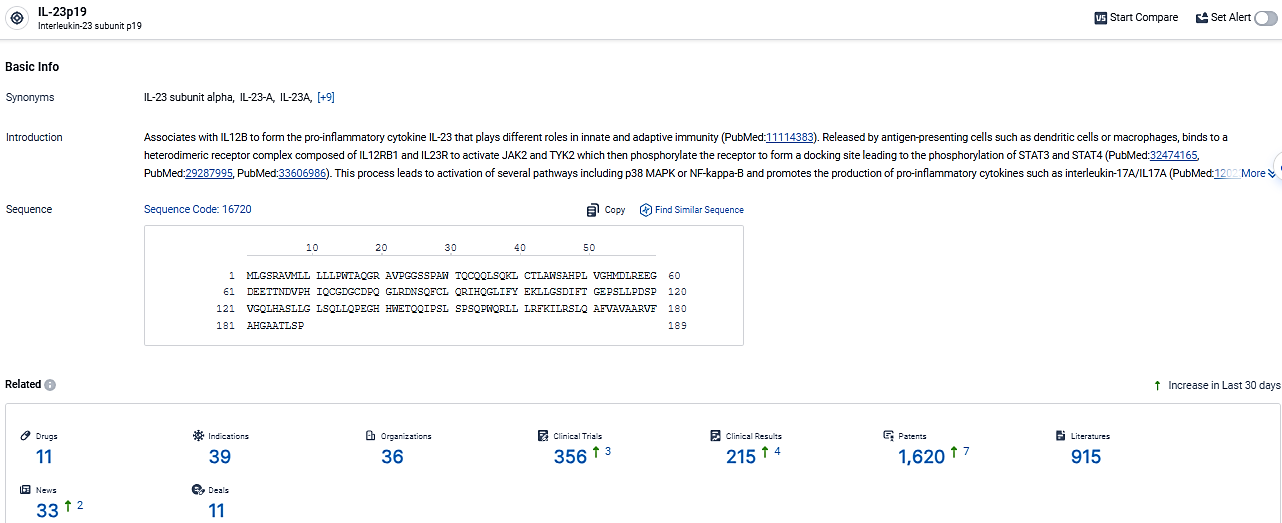
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 13, 2024, there are 11 investigational drugs for the IL-23p19 target, including 39 indications, 36 R&D institutions involved, with related clinical trials reaching 356, and as many as 915 patents.
TREMFYA® is the first approved fully-human, dual-acting monoclonal antibody that blocks IL-23 by binding to the p19 subunit of IL-23 and binding to CD64, a receptor on cells that produce IL-23. With its breakthrough therapy designation and ability to address multiple therapeutic areas, Guselkumab holds significant potential in the treatment of immune system diseases, infectious diseases, and various other conditions.