Several bispecific antibodies targeting GPCRs
Bispecific antibodies (bsAbs) possess unique advantages due to their ability to simultaneously target two distinct antigens, which helps to enhance the immune system's recognition and killing of tumor cells. As of the end of 2023, 14 bsAbs have been approved: 11 of them for cancer treatment and 3 for non-oncological indications. BsAbs come in various structural formats, target different antigens, and mediate antitumor functions through different molecular mechanisms.
The development of antibodies targeting G protein-coupled receptor (GPCR) targets is relatively more challenging compared to traditional drugs such as small molecules, peptides, recombinant hormones, etc., with slower progress in comparison.
In contrast, the development of Antibody-Drug Conjugates (ADCs) targeting members of the GPCR family has seen a gradual increase in research momentum over the past decade.
Dual-specificity antibodies related to GPCR
According to statistics from the Synapse database, there are currently 23 bispecific antibody therapeutics worldwide targeting GPCR (G protein-coupled receptor) class targets, including targets such as CXCR4 (+PD-L1) , GPRC5D (+CD3) , GPRC5D (+BCMA+CD30) , SSTR2 (+CD3) , ETA (+CD3) , ETA (+TGF-B) , CCR8 (+TIGIT) , CCR8 (+CTLA4) , CCR2 (+M-CSF) , FZD5 (+LRP6) , FZD4 (+LRP5), GIPR+GLP-1R, FSHR (+albumin) , LGR5 (+EGFR).
Johnson & Johnson's Talquetamab became the world's first approved bispecific antibody targeting a GPCR class target to be marketed. Bispecific antibodies that target both GPRC5D and CD3 include Nanjing Leads Biolabs' LBL-034, which is in Phase 1/2 clinical trials, and Roche's Forimtamig, which is in Phase 1 clinical development.
Additionally, the GPRC5D+CD3+BCMA trispecific antibody MBS314, co-developed by Kyinno Biotechnology and Beijing Mabworks Biotech, is in Phase 1/2 clinical trials, and the GPRC5D+CD3+BCMA trispecific antibody SIM-0500, co-developed by Simcere Pharmaceutical Group Limited and Shanghai Xianxiang Pharmaceutical Technology, is in the clinical application stage.
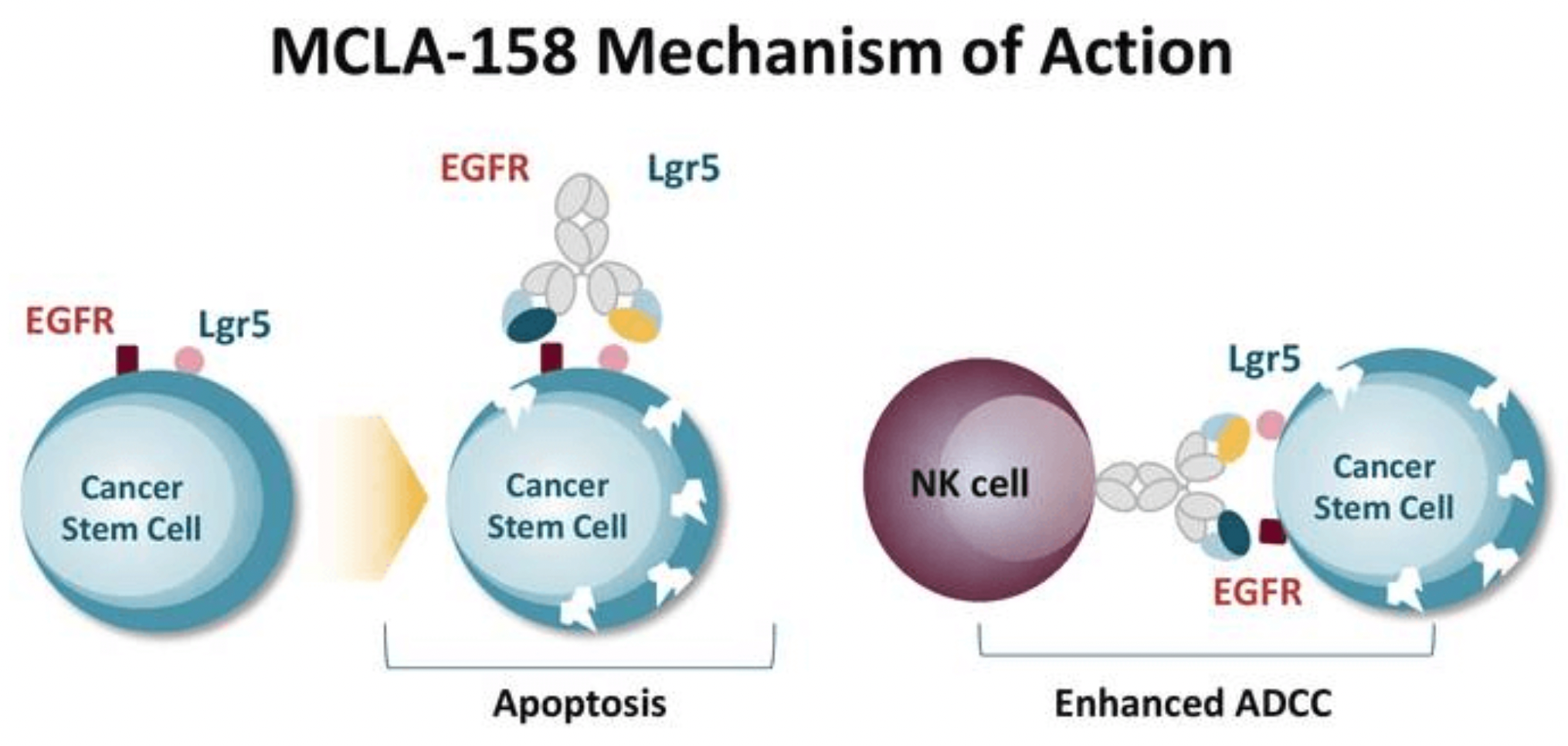
Translate the following text into English with professional accuracy: The Dutch biotechnology company Merus has developed a bispecific product, Petosemtamab (MCLA-158), currently in Phase 1/2 clinical trials. This is a bispecific Biclonics® fucose-low full human IgG1 antibody that simultaneously targets the epidermal growth factor receptor (EGFR) and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5). Petosemtamab is designed with three independent mechanisms of action:
Inhibition of the EGFR-dependent signaling pathway: By binding with EGFR on the surface of tumor cells, it blocks normal signal transmission, thereby inhibiting processes such as tumor cell proliferation, migration, and angiogenesis.
LGR5-mediated EGFR internalization and degradation: Petosemtamab can bind to LGR5, leading to the internalization and degradation of EGFR within cancer cells, further diminishing EGFR’s pro-carcinogenic activity.
Enhancement of antibody-dependent cellular cytotoxicity and phagocytosis (ADCC/ADCP): With its bispecific structure, Petosemtamab can recruit and activate the immune system's natural killer (NK) cells and macrophages, enhancing their ability to recognize and eliminate cancer cells expressing EGFR and LGR5, thus achieving indirect antitumor effects.
At the 2023 Annual Meeting of the American Association for Cancer Research (AACR), Merus presented the results of a Phase 1/2 clinical trial cohort expansion, showing that Petosemtamab demonstrated a manageable safety profile and significant therapeutic effects in patients with advanced head and neck squamous cell carcinoma (HNSCC). Among the 43 patients with advanced HNSCC treated with Petosemtamab, the overall response rate (ORR) reached 37.2%, with tumor reductions observed in 16 patients. The data specifics included: 1 patient achieved a complete response (CR), and 15 patients had partial responses (PR). In addition, 15 patients achieved a stable disease (SD) condition. As a result, the disease control rate (DCR) was 72.1% (n=31/43; 95% CI, 56.3%-84.7%), which means that over seventy percent of the patients experienced no tumor progression or saw reductions in tumor size following treatment.

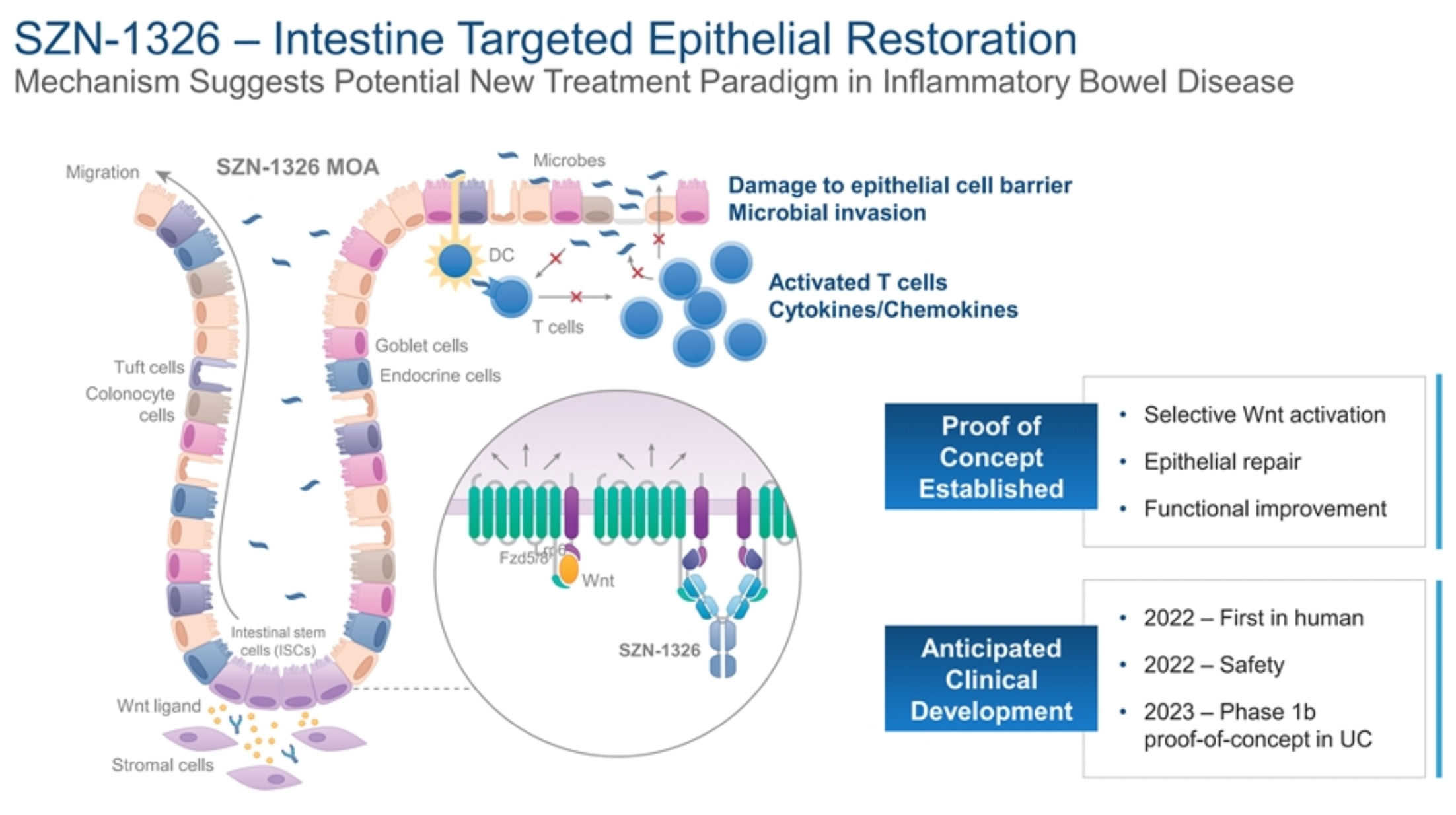
The bi-specific product SZN-1326, originally developed by the American biotechnology company Surrozen, is in Phase 1 clinical research. The product is designed based on Surrozen's proprietary SWAP™ technology, with the goal of modulating the Wnt signaling pathway in intestinal epithelium. The Wnt/β-catenin signaling pathway plays a crucial role in the renewal of intestinal stem cells, the maintenance of intestinal epithelial homeostasis, and the regeneration process. Studies have confirmed that this pathway is dysregulated in inflammatory bowel disease (IBD). Fzd5 is a Wnt receptor with high expression in intestinal epithelial tissues.
SZN-1326 is a bi-specific, effectorless IgG1 antibody that targets both FZD5 and 8, as well as the LRP6 receptor. Preclinical research has indicated that SZN-1326 can effectively activate the Wnt signaling pathway. Furthermore, in organoid cultures of the intestine, it can stimulate the growth of the organoids; it increases the expression levels of the Wnt target gene Axin2 in colonic tissue; and in a mouse model, SZN-1326 can also ameliorate colitis symptoms induced by Dextran Sulfate Sodium (DSS).
At present, bispecific antibodies targeting GPCR class targets are primarily indicated for cancer-related clinical applications, with popular therapeutic directions including multiple myeloma, plasma cell myeloma, solid tumors, and more. However, there is also a diversity of clinical indications, such as bispecific antibodies targeting GIPR and GLP-1R for the treatment of obesity and diabetes, bispecific antibodies targeting ETA for the treatment of pulmonary fibrosis and pulmonary arterial hypertension, bispecific antibodies targeting FSHR for the treatment of male/female infertility, and bispecific antibodies targeting FZD4 for the treatment of diabetic retinopathy and wet age-related macular degeneration.