Janssen Seeks Expanded Approval for Rybrevant In combination with Chemotherapy
Johnson & Johnson's Janssen Pharmaceutical Companies have submitted a supplemental Biologics License Application to the U.S. Food and Drug Administration. This is for expanded usage of RYBREVANT® (amivantamab-vmjw), along with chemotherapy (carboplatin-pemetrexed), as a primary method of treating local advanced or metastatic non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor exon 20 insertion mutations. The sBLA is currently under review by FDA's Real-Time Oncology Review (RTOR) program.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Kiran Patel, M.D., Vice President of Clinical Development for Solid Tumors at Janssen Research & Development, LLC, shed light on the PAPILLON Phase 3 clinical trial – the first of its kind to indicate results of clinical significance in NSCLC patients with EGFR exon 20 insertion mutations. She expressed that this presented a considerable chance to enhance the typical course of treatment for patients with this condition. Patel also stated that Janssen eagerly anticipates the opportunity to work with the RTOR program in seeking approval for RYBREVANT plus chemotherapy.
RYBREVANT® was bestowed the Status of Breakthrough Therapy by the U.S. FDA in 2020, after which it received a fast-track approval in 2021 as the premier fully-selective antibody for NSCLC patients with EGFR exon 20 insertion mutation treatment. The sBLA submission was aimed at fulfilling the regulatory mandates for this accelerated confirmation, highlighting the clinical benefit noted in the Phase 1 CHRYSALIS examination.
The sBLA relies on data from the randomized, open-label Phase 3 PAPILLON clinical trial that evaluated the efficacy and safety of RYBREVANT® in tandem with chemotherapy. Earlier in July, Janssen unveiled that the PAPILLON study managed to reach its primary endpoint showing marked improvement in PFS.
RYBREVANT® (amivantamab-vmjw) was expediently authorized for use by the FDA in May 2021. It was designed to remedy adult patients having locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations who exhibited disease progression following platinum-based chemotherapy.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer◊ recommend NGS-based methods over PCR-based techniques for recognizing EGFR exon 20 insertion variations and suggest amivantamab-vmjw (RYBREVANT®) as a following treatment choice with a Category 2A endorsement for those who have progressed either with or after platinum-based chemotherapy with or without immunotherapy and suffer from an EGFR exon 20 insertion mutation-positive advanced NSCLC.
In addition to the Phase 3 PAPILLON trial, RYBREVANT® is being examined in several clinical studies in NSCLC, including:
• The Phase 3 MARIPOSA (NCT04487080) study is looking into RYBREVANT® combined with lazertinib, a novel third generation EGFR TKI, putting them up against osimertinib and against lazertinib alone in the first-line treatment of patients suffering from locally advanced or metastatic NSCLC with EGFR exon 19 deletions (ex19del) or L858R substitution mutations.
• The Phase 3 MARIPOSA-2 evaluates the effectiveness of RYBREVANT® (used with or without lazertinib) and carboplatin-pemetrexed when compared to carboplatin-pemetrexed in patients having locally advanced or metastatic EGFR ex19del or L858R substitution NSCLC following disease progression with or after osimertinib.
• The Phase 1 CHRYSALIS trial inspects RYBREVANT® in individuals affected by advanced NSCLC.
• The Phase 1/1b CHRYSALIS-2 investigates RYBREVANT® used in tandem with lazertinib and lazertinib as a monotherapy in patients suffering from advanced NSCLC carrying EGFR mutations.
• The Phase 1 PALOMA trial determines the suitability of injecting amivantamab into the skin (SC) based on safety and pharmacokinetics with the aim of devising a dose, dosage schedule, and formulation for SC delivery.
• The Phase 2 PALOMA-2 looks into injecting amivantamab into the skin for participants carrying advanced or metastatic solid tumors including EGFR-mutated NSCLC.
• The Phase 3 PALOMA-3 compares lazertinib with amivantamab injected in the skin to amivantamab given intravenously in participants carrying EGFR-mutated advanced or metastatic NSCLC.
• The Phase 1/2 METalmark deliberates a combination of RYBREVANT® and capmatinib therapy for those suffering from locally advanced or metastatic NSCLC.
• The Phase 1/2 PolyDamas tests a combination of RYBREVANT® and cetrelimab therapy in patients with advanced or metastatic NSCLC.
• The Phase 2 SKIPPirr study tries out ways to minimize first-dose infusion related reactions with RYBREVANT® when combined with lazertinib in recurring or stubborn EGFR-mutated advanced or metastatic NSCLC.
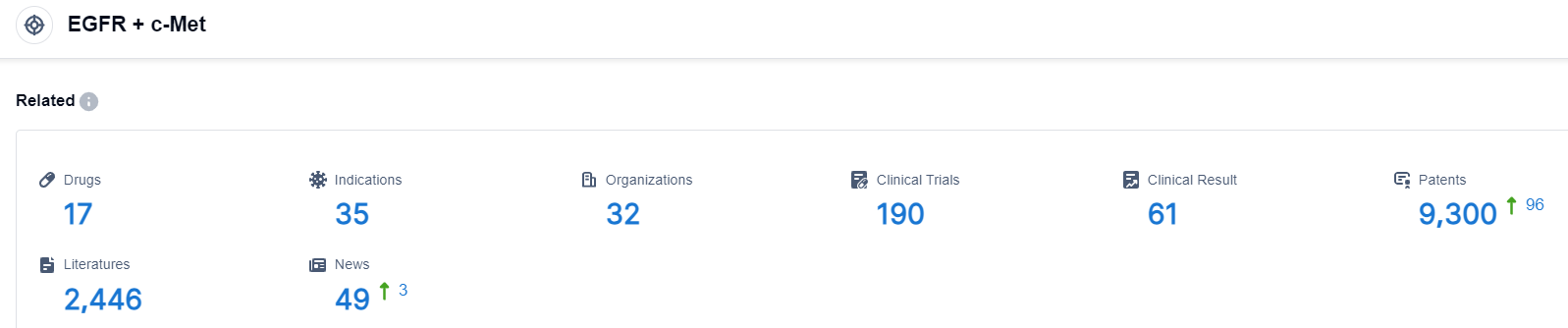
Click on the image below for direct access to the latest R&D progress on EGFR+c-Met target drugs, indications, research institutions, clinical trials, and more. as of August 30, 2023, there are a total of 17 drugs under research for the EGFR+c-Met target, covering 35 indications, with 32 research institutions involved, 190 related clinical trials, and as many as 9300 patents. The arrival and subsequent uptake of cheaper biosimilars and generics for several therapies will apply some downward pressure on the NSCLC market’s overall commercial potential, but this will be outweighed by the introduction and uptake of next-generation therapies and novel combinations. Avastin, Alimta, and Abraxane are now primarily used in combination with Keytruda and Tecentriq in the first-line setting, and the continued uptake of these checkpoint inhibitor regimens will balance out the downward pressure from the introduction of generics, particularly if future approvals allow for branded therapies like Lenvima to be added to the current regimens. Additionally, both Vargatef and Alimta are expected to be replaced in the second-line setting by pipeline drugs currently in development for the post-immunotherapy setting. Furthermore, Tagrisso has already supplanted Tarceva and Iressa inEGFR-positive NSCLC after demonstrating superiority over both drugs in the Phase III FLAURA trial.