KEYTRUDA® (pembrolizumab), a Merck product, met its main objective of disease-free survival (DFS) in specific patients with Muscle-Invasive Urothelial Carcinoma post-surgery
Merck, recognized as MSD beyond U.S. and Canada, recently proclaimed that the Phase 3 AMBASSADOR study assessing KEYTRUDA, an anti-PD-1 treatment developed by Merck, achieved one of its two major outcomes related to disease-free survival for supplemental treatment of individuals suffering from muscle-invasive urothelial carcinoma localized or urothelial carcinoma locally advanced compared to monitoring
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Analyzing results from an explicitly planned interim analysis review undertaken independently by the Data Monitoring Committee, KEYTRUDA showcased a meaningful, and statistically significant, enhancement in DFS compared to observation in patients post-surgery. This trial is still under progress to assess its dual primary endpoint of total survival.
In this specific trial, the safety characteristics of KEYTRUDA matched those observed in previous studies, with no new safety indicators noted. The data will be discussed at an imminent medical gathering and deliberated with regulatory officials.
“Roughly 50% of bladder cancer patients who go through surgeries will see a relapse within a year, emphasizing the urgency for new therapeutic alternatives in the adjuvant environment,” noted Dr. Marjorie Green, who is the senior vice president and in charge of late-stage oncology at global clinical development, Merck Research Laboratories. “The encouraging results accentuate KEYTRUDA's ability to prevent recurrence post-surgery for those with muscle-invasive or locally advanced urothelial carcinoma.”
This study was financially supported by the U.S. National Cancer Institute, a fraction of the National Institutes of Health. Alliance for Clinical Trials in Oncology composed and directed the trial with backing from the NCI and the involvement of all the National Clinical Trials Network Groups. Merck also offered funding and support via a Cooperative Research and Development Agreement with NCI.
Merck's extensive clinical development program is testing KEYTRUDA both as a standalone solution and in conjunction with other anti-cancer treatments for every stage of bladder cancer, including non-muscle-invasive, muscle-invasive and metastatic phases. Late-stage trials in muscle-invasive bladder cancer encompass the KEYNOTE-866 examination, combined with the Phase 3 KEYNOTE-B15 and Phase 3 KEYNOTE-905 studies, undertaken in partnership with Seagen and Astellas.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
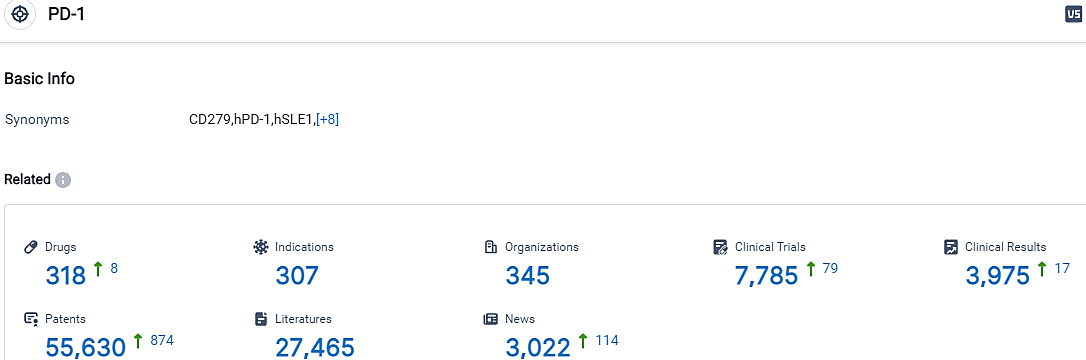
According to the data provided by the Synapse Database, As of October 17, 2023, there are 318 investigational drugs for the PD-1 target, including 307 indications, 345 R&D institutions involved, with related clinical trials reaching 7785,and as many as 55630 patents.
KEYTRUDA operates as an anti-programmed death receptor-1 treatment, enhancing the body's immune defense to identify and combat cancer cells. Characterized as a humanized monoclonal antibody, KEYTRUDA impedes the communication between PD-1 and its corresponding ligands, PD-L1 and PD-L2. By doing so, it invigorates T lymphocytes which could impact not only cancer cells but normal cells too.