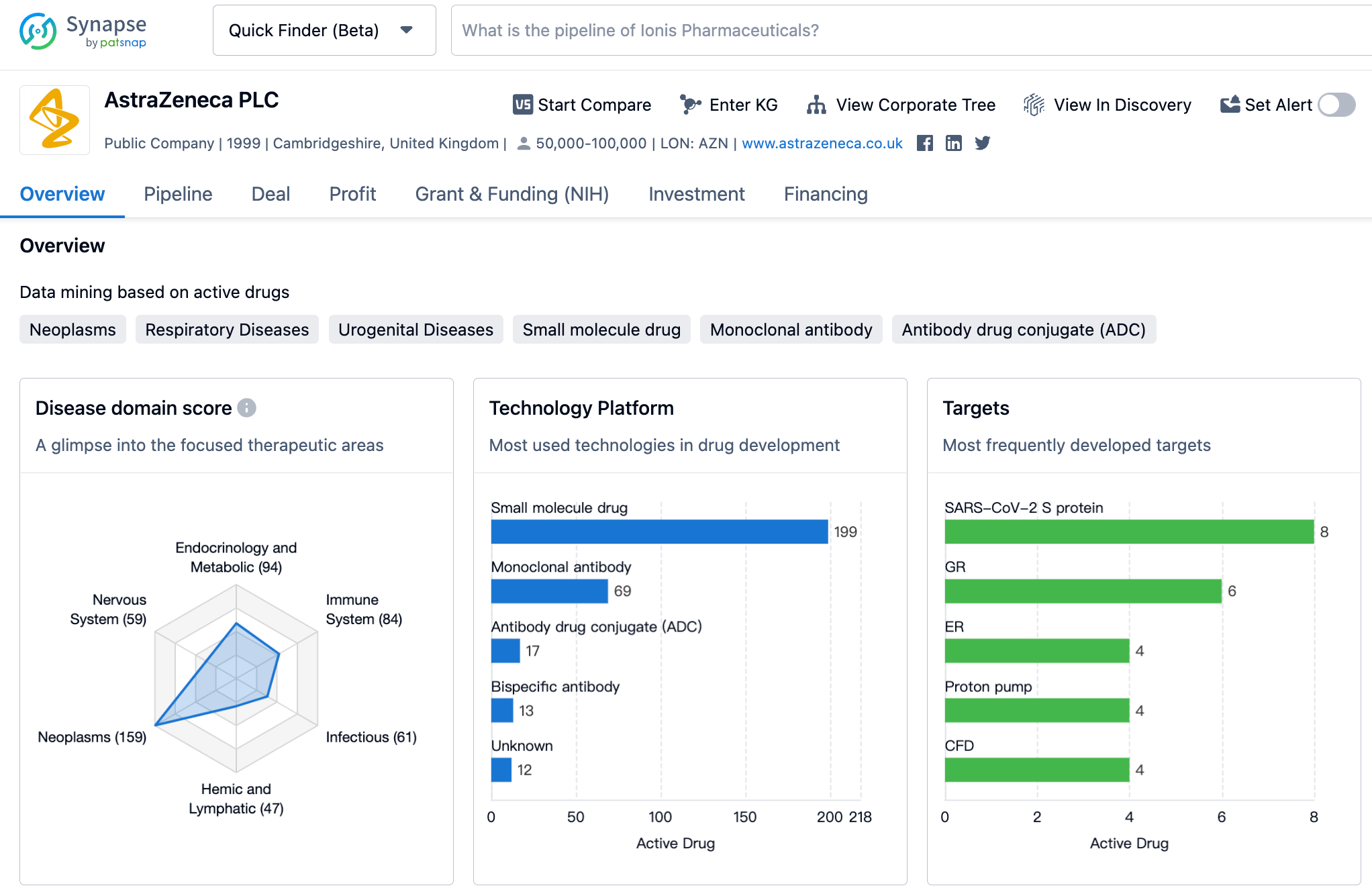
Latest Competitive Analysis of AstraZeneca Drug Pipeline
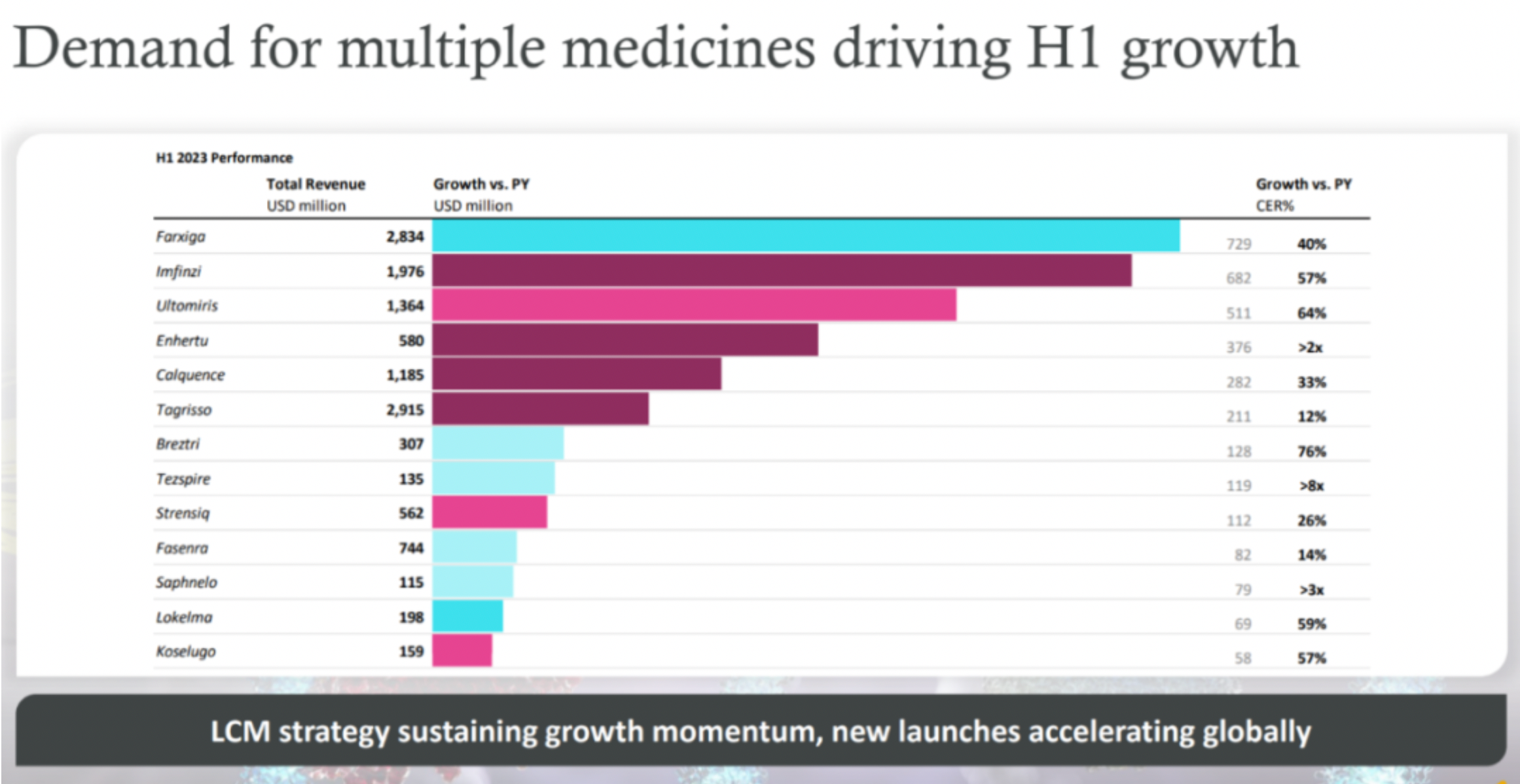
AstraZeneca has shown a steady performance in the past two years, and its market value has effectively stabilized at over 200 billion. Despite the significant impact of COVID-19 vaccines and neutralizing antibodies going nearly to zero, the company's performance remained stable, principally due to colossal increments contributed by Dapagliflozin, Durvalumab, Ravulizumab, Enhertu, etc.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of AstraZeneca.
Advancements on multiple fronts in cardiovascular, oncology, and rare diseases further underscore AZ's comprehensive advantages post its recent turnaround.
Their research and development progress is one of the most balanced and conspicuous amongst major pharmaceutical companies.

Aside from several important oncology products tirelessly making new breakthroughs, notable progress includes the submission of an NDA for the Akt inhibitor Capivasertib used in conjunction with Fulvestrant for HR+ breast cancer treatment, the publication of phase one clinical results of DS-1062 for 1L NSCLC treatment, the approval of Dapagliflozin for HFpEF indication, the release of phase three clinical data for the CFD medication Danicopan in the treatment of PNH, and the release of phase three clinical data for the ASO drug Eplontersen for the treatment of ATTR-PN. However, the development of Brazikumab, an IL-23 monoclonal antibody for IBD treatment, was discontinued.