Kite and Arcellx Propel Forward in Developing Anito-Cel for Multiple Myeloma Treatment Program
Kite, a subsidiary of Gilead Sciences, along with Arcellx, Inc., has provided numerous important updates regarding their collaborative project on anitocabtagene autoleucel (anito-cel) for treating multiple myeloma. Anito-cel represents the inaugural BCMA CAR T-cell therapy being studied in multiple myeloma cases, incorporating the innovative and efficient D-Domain binding technology from Arcellx.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Kite has disclosed the framework for a worldwide, Phase 3 randomized controlled clinical trial called iMMagine-3, slated to commence in the latter part of this year. This clinical study aims to evaluate the effectiveness and safety of anito-cel in comparison to the standard of care for patients with relapsed and/or refractory multiple myeloma. Patients eligible for this study should have undergone one to three previous therapy lines that included both an immunomodulatory agent and an anti-CD38 monoclonal antibody.
The production of anito-cel for this specific trial will occur at Kite's facility located in Frederick, Maryland. This comes after the successful technical handover of production capacities from an external contract manufacturing organization to Kite and the transition of the Investigational New Drug application for anito-cel, which received approval from the U.S. Food and Drug Administration.
Cindy Perettie, Executive Vice President at Kite, expressed her enthusiasm about launching the iMMagine-3 trial later this year, emphasizing the significant need for new therapeutic options for patients with this form of multiple myeloma. She stated, “As we gear up for this critical trial, we are eager to utilize our production capabilities to establish anito-cel as a leading contender in cell therapy. We recognize that the quality, dependability, and efficiency of manufacturing are paramount since timing is crucial for these patients.”
The trial's primary goal is to measure progression-free survival as determined by an independent blinded review, using the 2016 IMWG uniform response criteria for multiple myeloma. The hypothesis is that anito-cel will extend PFS compared to standard care. Important secondary objectives include assessing the complete response rate, the rate of minimal residual disease negativity, overall survival, and evaluating safety profiles.
Anito-cel, a BCMA CAR T-cell therapy, incorporates a novel binder known as the D-Domain. The notably small size of the D-Domain (8 kDa) enhances T-cell transduction and expression, leading to a higher number of CAR positive cells and increased CAR expression per T-cell. The therapy has received the Fast Track, Orphan Drug, and Regenerative Medicine Advanced Therapy designations from the U.S. Food and Drug Administration.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
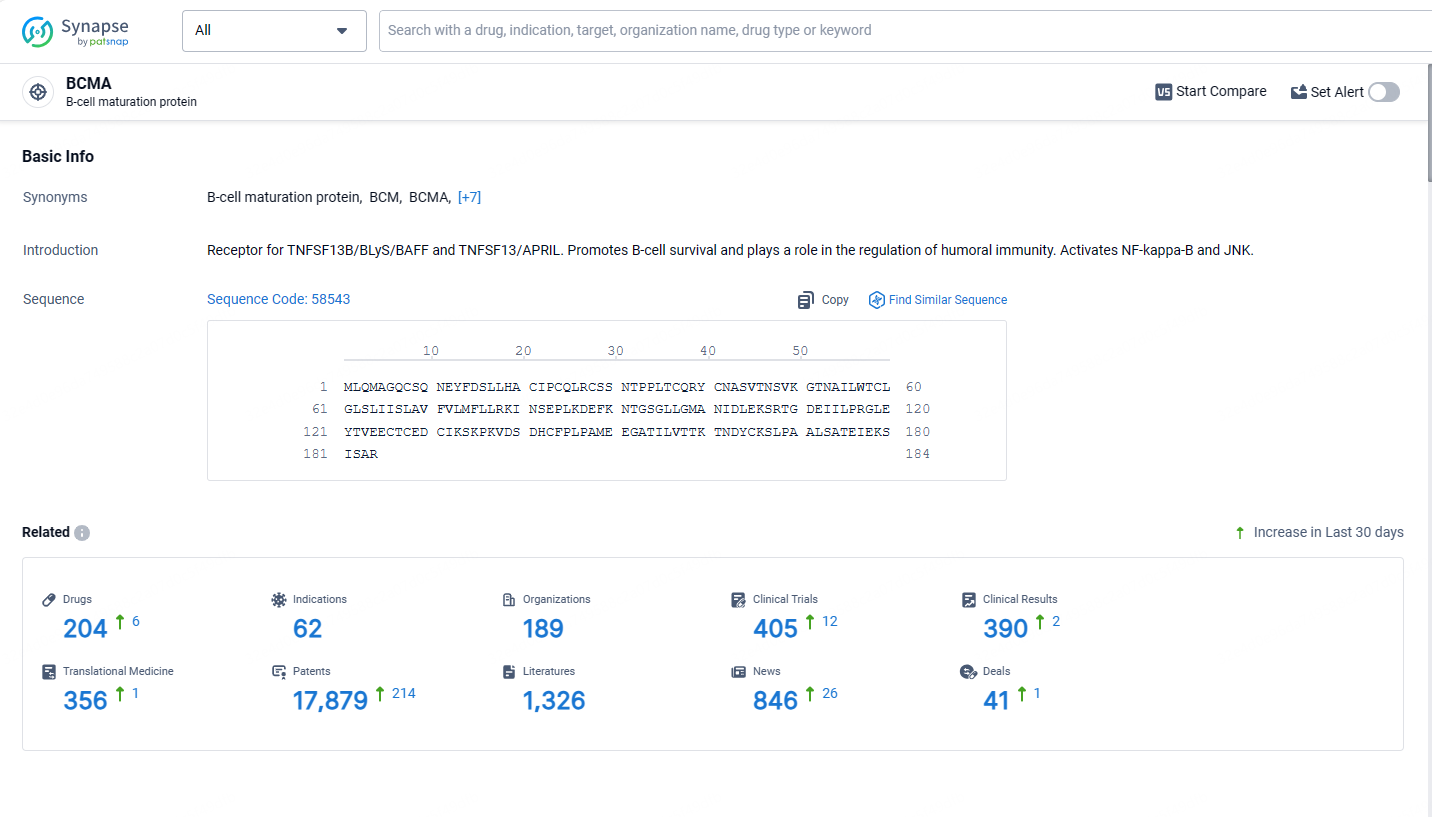
According to the data provided by the Synapse Database, As of May 13, 2024, there are 204 investigational drugs for the BCMA target, including 62 indications, 189 R&D institutions involved, with related clinical trials reaching 405, and as many as 17879 patents.
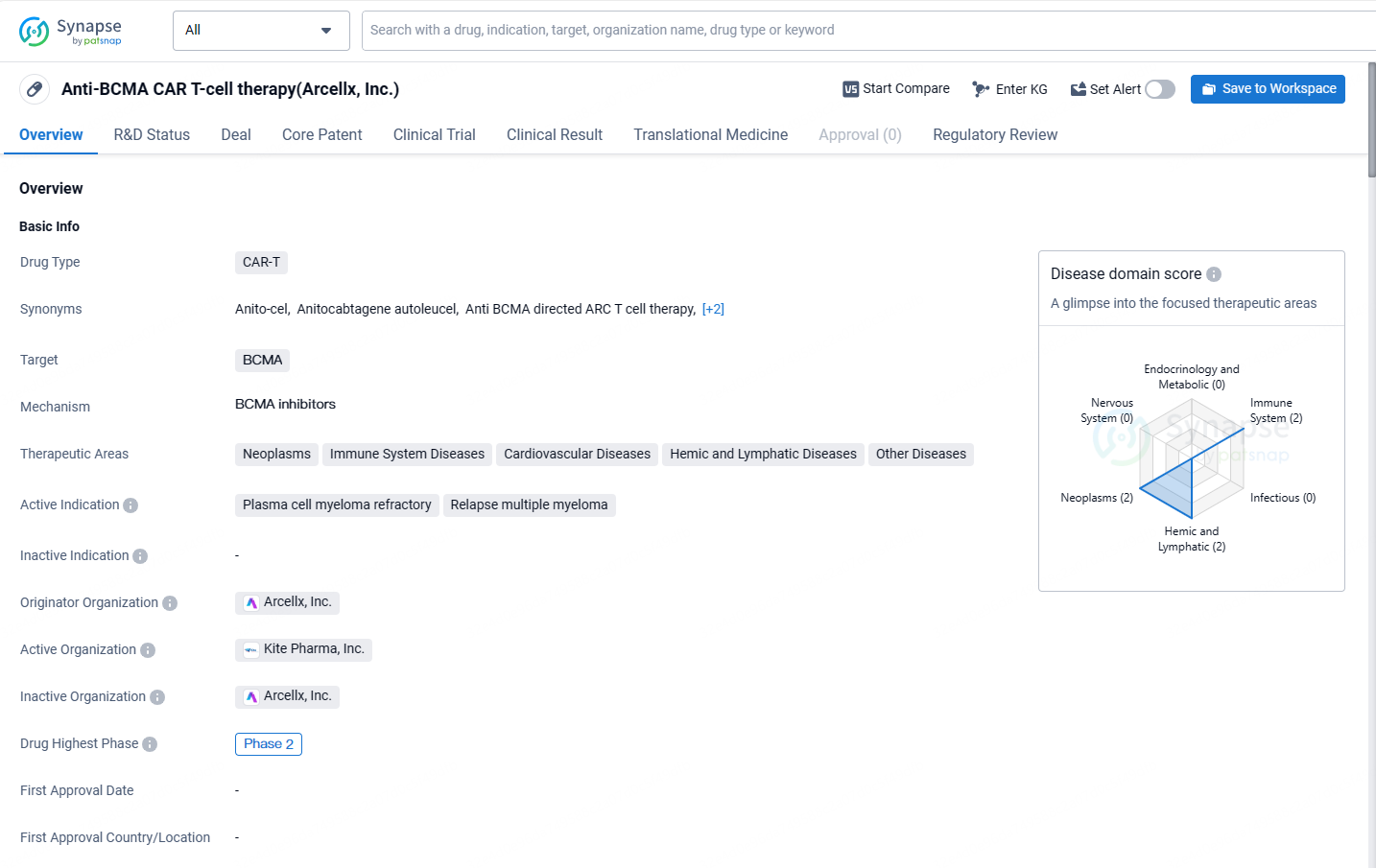
Anito-cel is a CAR-T drug that targets BCMA and is primarily indicated for refractory plasma cell myeloma and relapsed multiple myeloma. It is currently in Phase 2 of development and has received regulatory designations to facilitate its development and approval process. The drug also shows potential for treating other diseases within the therapeutic areas of neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, and other diseases.