Freeline Reveals Promising FLT201 Gene Therapy Results for Gaucher Disease at ASGCT's 27th Meeting
Freeline Therapeutics disclosed new findings from the Phase 1/2 GALILEO-1 study concerning FLT201, its gene therapy candidate for Gaucher disease, developed through adeno-associated virus technology. The data highlights significant decreases in glucosylsphinogsine, a key indicator of clinical outcomes, among patients whose levels remained elevated despite prolonged therapy with existing approved treatments. Additionally, preliminary observations suggest improvements in bone marrow burden and reductions in fatigue in these patients.
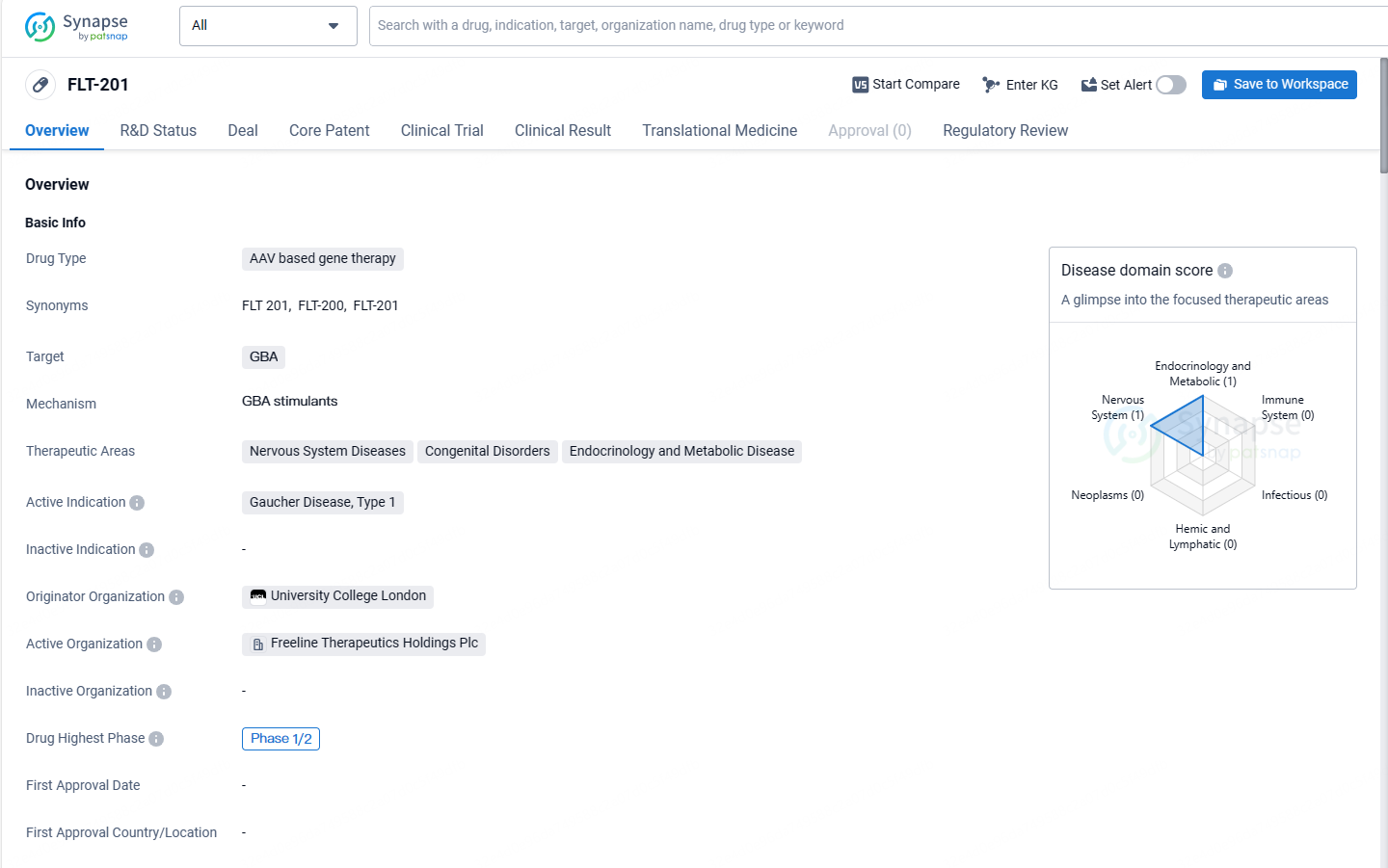
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The investigational gene therapy FLT201 is being presented at the American Society of Gene and Cell Therapy 27th Annual Meeting in Baltimore, Maryland, highlighting its promising safety and tolerability results through a key late-breaking oral presentation.
Gaucher disease stems from a mutation in the GBA1 gene, resulting in a deficit of the glucocerebrosidase enzyme. This deficiency leads to the accumulation of substrates in various cells and organs, manifesting in symptoms like spleen and liver enlargement, decreased blood cell counts, bone pain, fatigue, and impaired lung function.
The therapy FLT201 offers a specially designed version of the GCase enzyme that is more stable than its wildtype counterpart. It is engineered to remain within cells for extended periods, enhancing the breakdown and removal of substrates and effectively reaching areas like bone tissue—areas which are inadequately served by existing treatments. Improvements in the levels of lyso-Gb1 in the blood reflect reduced substrate presence in affected tissues and are indicative of favorable clinical results for Gaucher disease patients.
Ozlem Goker-Alpan, M.D., Founder and CEO of the Lysosomal and Rare Disorder Research and Treatment Center and a lead researcher in the FLT201 Phase 1/2 GALILEO-1 trial, stated, "Gaucher disease is the most prevalent lysosomal storage disorder and progresses severely if left untreated. Although existing therapies have significantly helped those affected by Gaucher disease, they fall short of a cure. Continuous life-long treatment is necessary, and patients often still suffer from symptoms like organ enlargement, ongoing bone discomfort, and fatigue."
FLT201, a gene therapy candidate based on an adeno-associated virus, is currently under study in the Phase 1/2 GALILEO-1 clinical trial for adults with Gaucher disease Type 1. It aims to durably enhance glucocerebrosidase levels and mitigate the buildup of detrimental substrates. The goal of FLT201 treatment is to offer a one-time intervention potentially halting disease progression, improving patient outcomes, and eliminating the need for continuous treatment. This innovative therapy utilizes
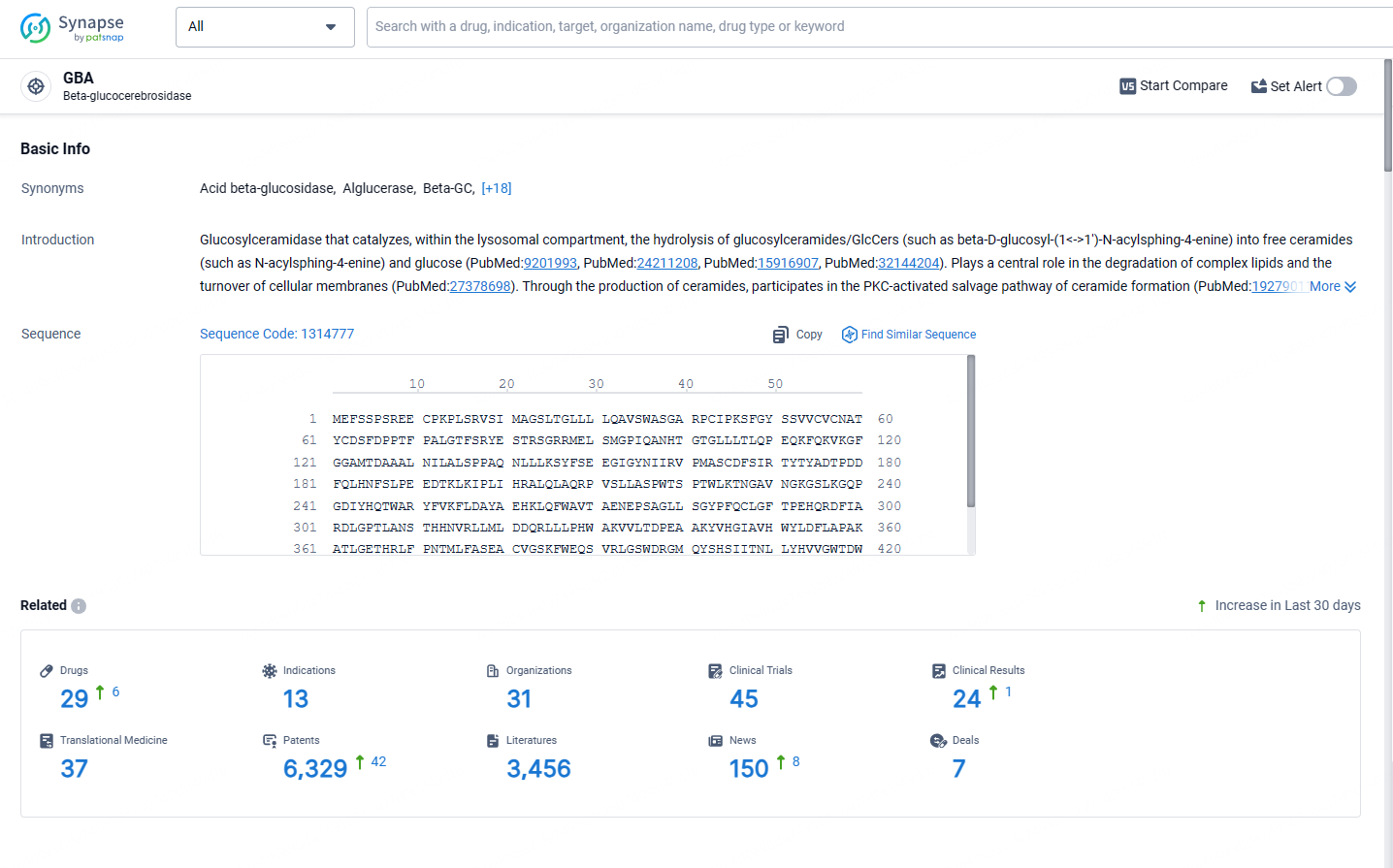
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 13, 2024, there are 29 investigational drugs for the GBA target, including 13 indications, 31 R&D institutions involved, with related clinical trials reaching 45, and as many as 6329 patents.
FLT-201 shows promise as a potential treatment for Gaucher Disease, Type 1. Its AAV-based gene therapy approach and orphan drug status highlight its innovative nature and potential benefits for patients with this rare genetic disorder. As the drug progresses through clinical trials, further data and analysis will be needed to assess its safety, efficacy, and potential for broader therapeutic applications.