Fate Therapeutics Begins Phase 1 Trial of FT819 CAR T-cell Therapy in Lupus Patient
Fate Therapeutics, Inc., a biopharmaceutical firm at the clinical phase, committed to developing a pioneering range of cellular immunotherapies derived from induced pluripotent stem cells (iPSC) for cancer and autoimmune diseases, recently disclosed that its Phase 1 autoimmunity trial of FT819 has commenced with the treatment of the initial patient diagnosed with systemic lupus erythematosus. FT819 represents the company’s readily available, CD19-targeted chimeric antigen receptor T-cell therapy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Furthermore, at the 27th Annual Meeting of the American Society of Gene and Cell Therapy, the organization disclosed translational outcomes from the preliminary FT819 Phase 1 trial addressing relapsed/refractory B-cell disorders, along with primary clinical findings from their Phase 1 evaluation of the FT522 universal, CD19-targeted CAR NK cell strategy in relapsed/refractory B-cell lymphoma. These findings underscore the scientific justification and elucidate crucial therapeutic activity mechanisms for managing B cell-driven autoimmune conditions.
The ongoing multi-site Phase 1 study of FT819 aims to evaluate its safety, pharmacokinetic properties, and its effectiveness against B cells in individuals suffering from moderate-to-severe systemic lupus erythematosus (SLE). The inaugural participant in this study, a 27-year-old female diagnosed with SLE for over a decade and unresponsive to multiple conventional therapies, was treated with conditioning chemotherapy prior to receiving a single infusion of 360 million FT819 cells.
Following a brief three-day hospitalization, the patient was released without experiencing any significant adverse effects. In a pioneering translational analysis utilizing a sample of her blood drawn before the chemotherapy, the FT819 showcased quick and potent elimination of the patient's CD19+ B cells in an ex vivo cytotoxic assay.
Jennifer Medlin, M.D., the Lead Investigator at the University of Nebraska Medical Center, expressed enthusiasm about the groundbreaking results from autologous CAR-T cell therapies that have shown early and enduring remissions in various B cell-mediated autoimmune conditions. She noted the aspiration to offer innovative, disease-altering treatments to patients.
Scott Wolchko, President and CEO of Fate Therapeutics, also conveyed excitement about advancing their iPSC-derived therapy platforms and pipeline candidates directed towards autoimmune diseases. He highlighted that preclinical and translational studies confirm the essential therapeutic action mechanisms of their ready-to-use FT819 CAR T-cell and FT522 CAR NK cell therapies for treating autoimmunity.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
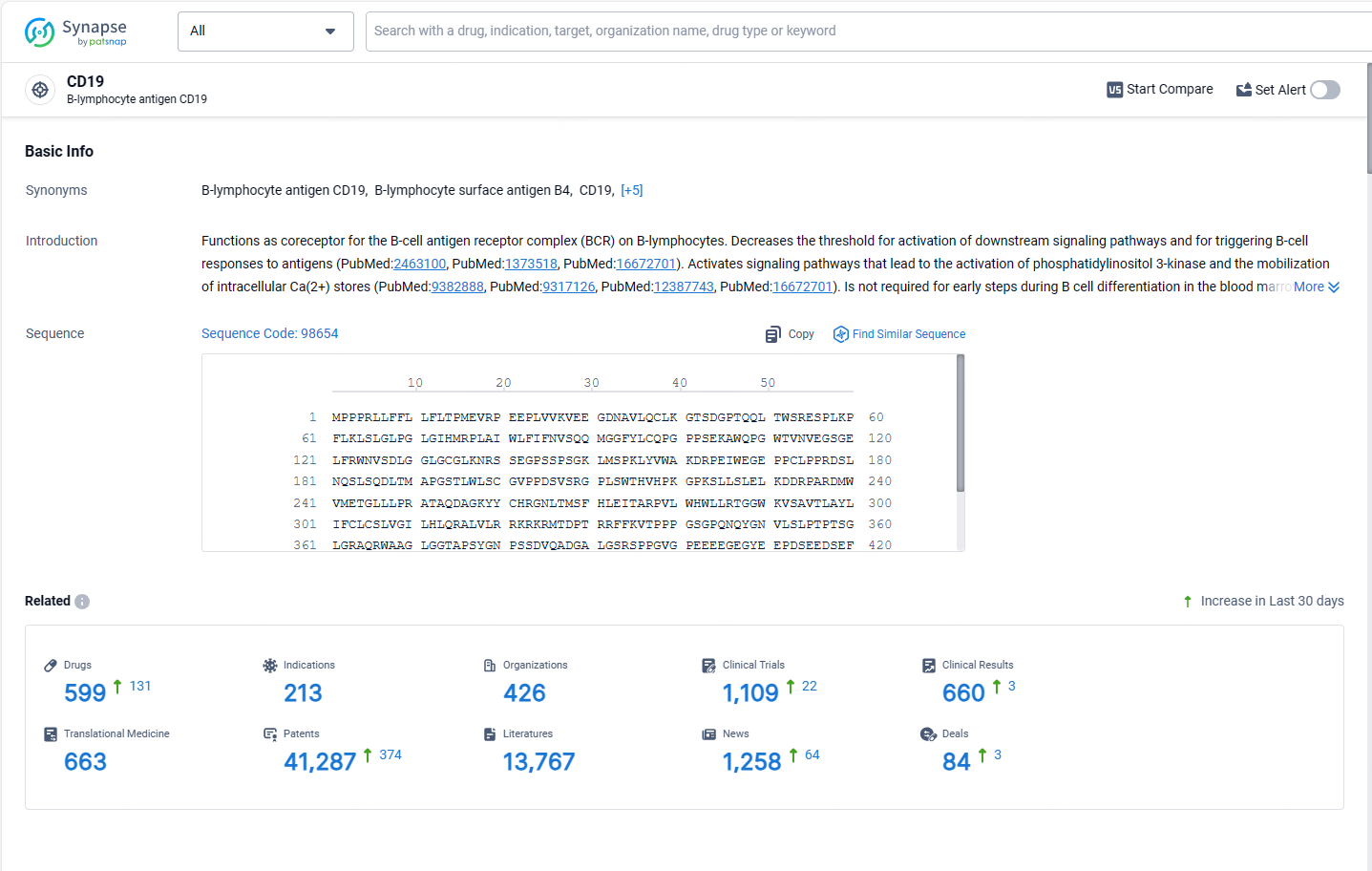
According to the data provided by the Synapse Database, As of May 13, 2024, there are 599 investigational drugs for the CD19 target, including 212 indications, 421 R&D institutions involved, with related clinical trials reaching 1109, and as many as 41252 patents.
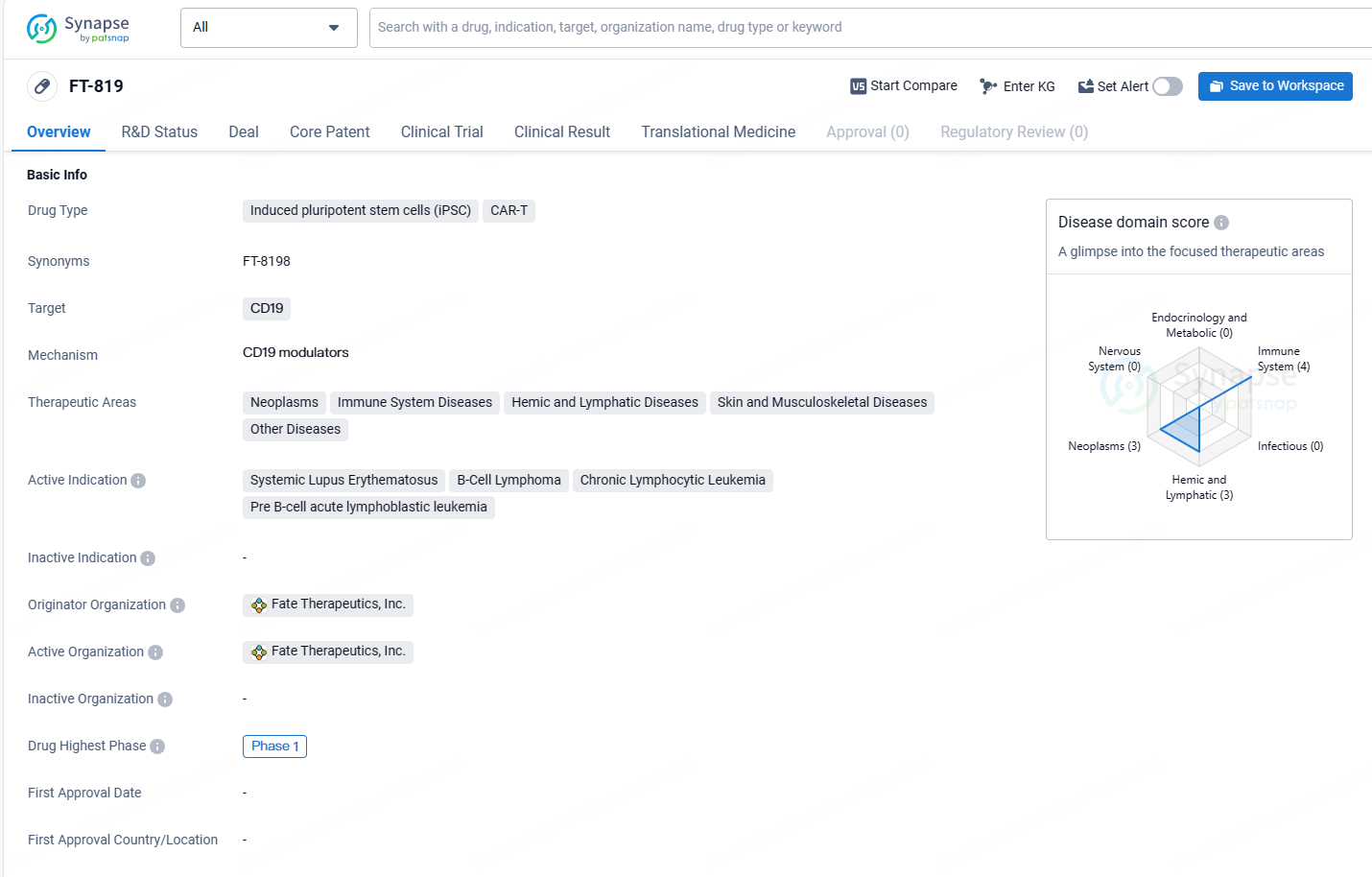
FT-819 targets CD19 and has potential applications in various therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and other diseases. The drug is currently in Phase 1 of clinical development and is being investigated for its efficacy in treating systemic lupus erythematosus, B-cell lymphoma, chronic lymphocytic leukemia, and pre B-cell acute lymphoblastic leukemia.