Kither Biotech Initiates Phase 1 Trial of KIT2014, a New Inhaled Peptide Therapy for Cystic Fibrosis
Kither Biotech, a biotech firm in the clinical development phase specializing in new treatments for respiratory disorders, has announced the initiation of a Phase 1 clinical trial for KIT2014, an innovative inhaled peptide treatment for Cystic Fibrosis (CF). This therapy is intended to complement current CFTR modulators, functioning by improving CFTR gating, facilitating bronchodilation, and decreasing inflammation via PDE3/4 inhibition.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The launch of this Phase 1 trial represents an important advancement for Kither Biotech and the cystic fibrosis (CF) community," stated Dr. Dimitrios Goundis, the CEO of Kither Biotech. "KIT2014 targets mucus buildup, chronic inflammation, and bronchoconstriction-three key issues that persist in CF patients even with existing CFTR modulators. We are eager to evaluate the potential of KIT2014 to enhance treatment results."
Overview of the KIT2014 Trial
This Phase 1 double-blind, placebo-controlled trial aims primarily to assess the safety and tolerability of both single and multiple doses of KIT2014 in healthy adult participants, followed by a pharmacokinetic evaluation. Further details can be found at clinicaltrials.gov (NCT06659757).
Understanding Cystic Fibrosis
Cystic fibrosis is a genetic disorder with life-threatening implications, affecting around 130,000 individuals globally. It results from mutations in the CFTR gene, leading to dysfunctional chloride ion transport across cell membranes and causing the accumulation of thick, viscous mucus in the lungs. This mucus obstructs mucociliary clearance, fosters chronic bacterial infections, and instigates continuous inflammation, leading to progressive lung deterioration.
The Medical Need for KIT2014
While advancements in CFTR modulators have been significant, many individuals with cystic fibrosis still endure ongoing inflammation and recurrent infections, which severely compromise lung function and overall well-being. Neutrophil-driven inflammation, characterized by increased neutrophil elastase levels, is a significant factor contributing to lung damage in CF patients. KIT2014 provides an innovative approach to address these challenges by specifically targeting this inflammation, minimizing neutrophil activation, and offering additional therapeutic benefits alongside current CF treatments. KIT2014 has the potential to greatly enhance long-term outcomes for individuals with cystic fibrosis.
Action Mechanism of KIT2014
KIT2014 is a peptide-based treatment designed to tackle these core problems by influencing multiple pathways involved in CF lung disease. Administered via inhalation, it enables direct targeting of lung tissue. By inhibiting phosphodiesterase 3/4 (PDE3/4), it increases levels of cyclic adenosine monophosphate (cAMP). This action enhances CFTR opening and improves chloride ion transport in bronchial epithelial cells, while also functioning as a bronchodilator in airway smooth muscle cells. Moreover, KIT2014 mitigates neutrophil-driven inflammation through PDE3/4 inhibition. Collectively, these mechanisms make KIT2014 a promising complementary therapy to existing CFTR modulator treatments.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
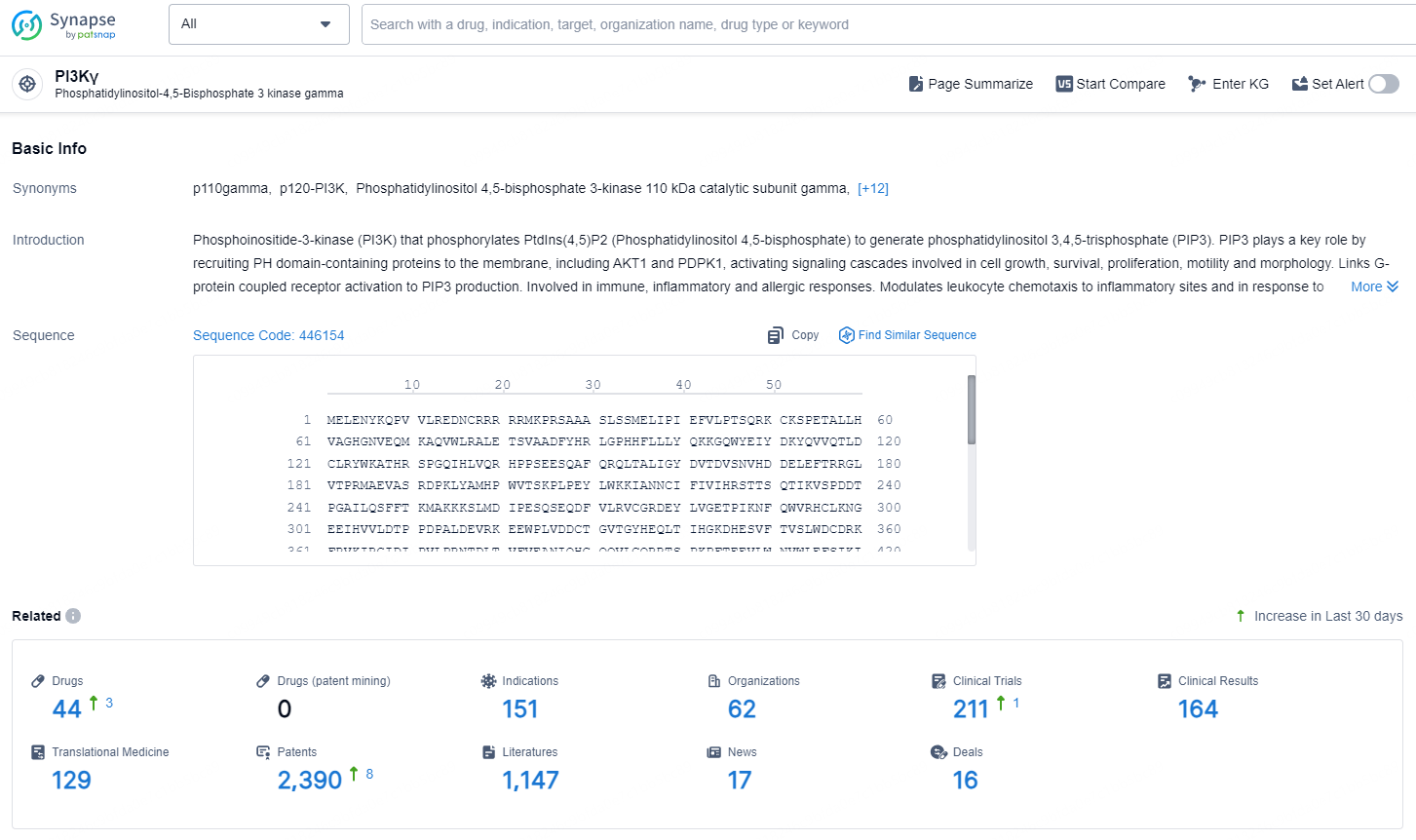
According to the data provided by the Synapse Database, As of November 5, 2024, there are 44 investigational drugs for the PI3Kγ target, including 151 indication, 62 R&D institutions involved, with related clinical trial reaching 211, and as many as 2390 patents.
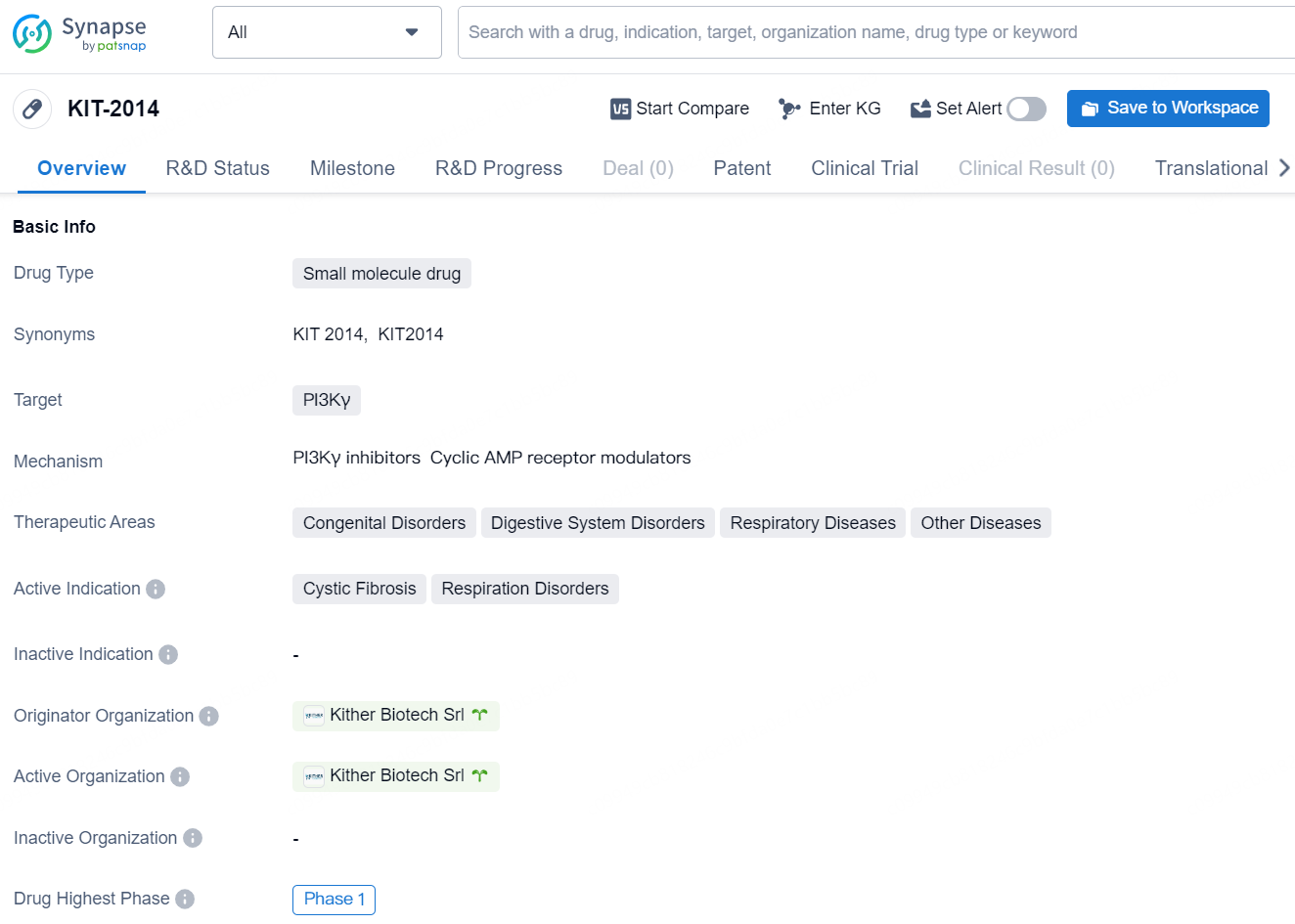
The drug KIT-2014 is a small molecule drug that targets PI3Kγ, and it falls within the therapeutic areas of congenital disorders, digestive system disorders, respiratory diseases, and other diseases. Its active indication includes cystic fibrosis and respiratory disorders. The originator organization of KIT-2014 is Kither Biotech Srl.