Kymera Therapeutics Reveals New Phase 1 Trial Data for KT-333 at EHA Event
Kymera Therapeutics, Inc., a biopharmaceutical company in the clinical stage that focuses on developing a novel category of small molecule drugs via targeted protein degradation, revealed new clinical data from its ongoing Phase 1 trial of KT-333. KT-333, a pioneering and powerful heterobifunctional small molecule degrader specifically targeting STAT3, showed antitumor activity in hematological cancers with significant unmet needs, such as relapsed/refractory classic Hodgkin's lymphoma, cutaneous T-cell lymphoma, and NK-cell lymphoma, at well-tolerated dosage levels.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The complete responses observed in Hodgkin’s lymphoma highlight the transformative therapeutic potential of STAT3 degradation," stated Jared Gollob, MD, Chief Medical Officer at Kymera Therapeutics. "Within the KT-333 trial, two extensively pretreated cHL patients transitioned to potentially curative stem cell transplants post-treatment.
The ongoing Phase 1 trial continues to provide encouraging data, demonstrating the clinical translation of our degrader's profile across various hematological malignancies, including cHL, CTCL, and NK-cell lymphoma, potentially bettering patients' lives. We anticipate completing the dose escalation phase and sharing the comprehensive dataset later this year."
The Phase 1 KT-333 clinical trial is assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of KT-333 administered weekly in 28-day cycles to adult patients with relapsed and/or refractory lymphomas, leukemias, and solid tumors.
In cHL, complete responses were achieved in two out of three patients at DL4. These responders, previously treated with at least one regimen including a checkpoint inhibitor and brentuximab vedotin, subsequently underwent stem cell transplantation. Additionally, stable disease was recorded in another cHL patient at DL6.
For NK-cell lymphoma, one patient with a STAT3 mutation achieved a complete response at DL7. Among nine CTCL patients, partial responses were seen in four patients at DL2 and DL4-6, with stable disease in one patient at DL4.
Furthermore, induction of an IFN-γ stimulated gene signature, predictive of anti-PD1 sensitivity, was detected in both peripheral blood and tumor, suggesting a favorable immunomodulatory response in the tumor microenvironment post-KT-333 treatment. This supports potential novel combinations with anti-PD1 drugs in solid tumors.
KT-333 was generally well tolerated, with most adverse events being Grade 1 and 2, including stomatitis and fatigue. Dose-limiting toxicities of Grade 3 stomatitis and arthralgia occurred in LGL-L patients at DL5, and Grade 3 fatigue was observed in a lymphoma patient at DL7."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
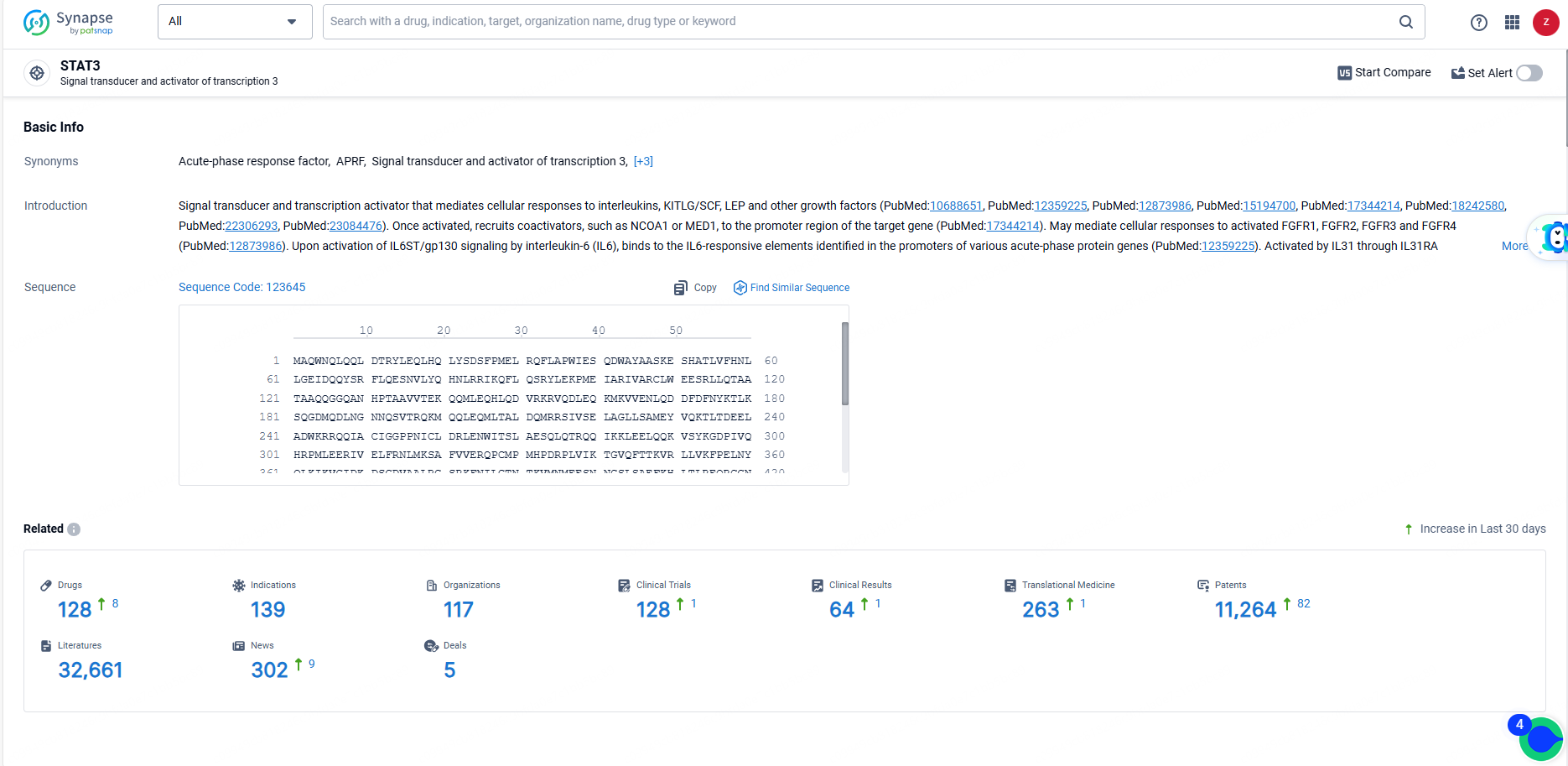
According to the data provided by the Synapse Database, As of June 19, 2024, there are 128 investigational drugs for the STAT3 targets, including 139 indications, 117 R&D institutions involved, with related clinical trials reaching 128, and as many as 11264 patents.
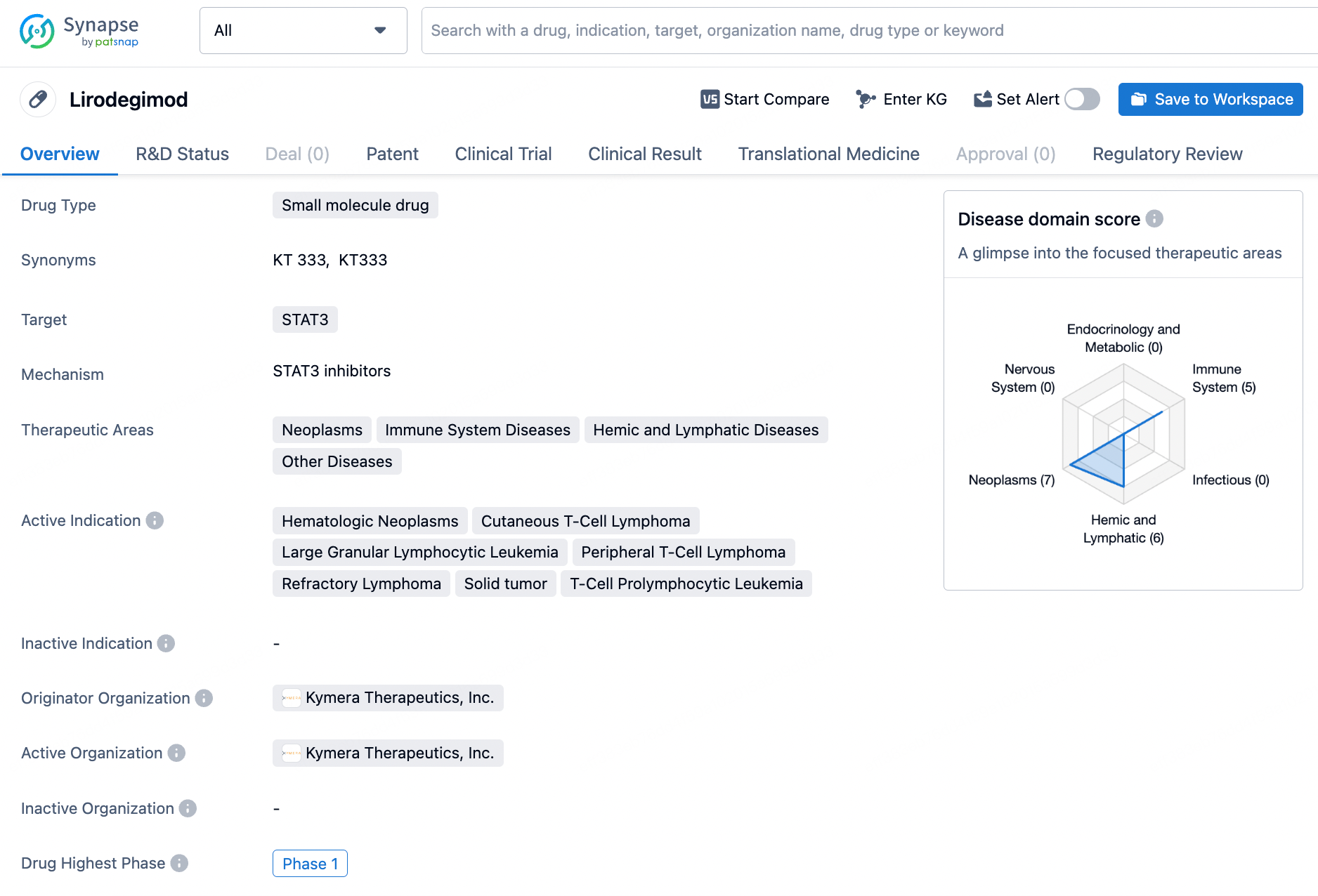
Lirodegimod shows promise as a potential treatment for a variety of serious and rare diseases, and its Fast Track and Orphan Drug designations indicate that it may address unmet medical needs in these therapeutic areas. As the drug progresses through clinical development, further research and testing will be necessary to determine its safety and efficacy for the indicated conditions.