LENZ Therapeutics Submits NDA to FDA for Novel Eye Drop Treatment for Presbyopia
On August 12, LENZ Therapeutics, a biopharmaceutical company dedicated to developing the first and only eye drop based on aceclidine (LNZ100) to improve near vision in presbyopia patients, announced that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for the treatment of presbyopia.
It is estimated that approximately 1.8 billion people globally and 128 million in the United States are affected by presbyopia, with a potential market size of about $3 billion.
Aceclidine is a non-selective muscarinic acetylcholine receptor (mAChRs) agonist. In the recently published Phase III CLARITY study, LNZ100 achieved all primary and secondary endpoints for near vision improvement and demonstrated statistically significant improvements of three lines or more in best-corrected distance visual acuity (BCDVA).
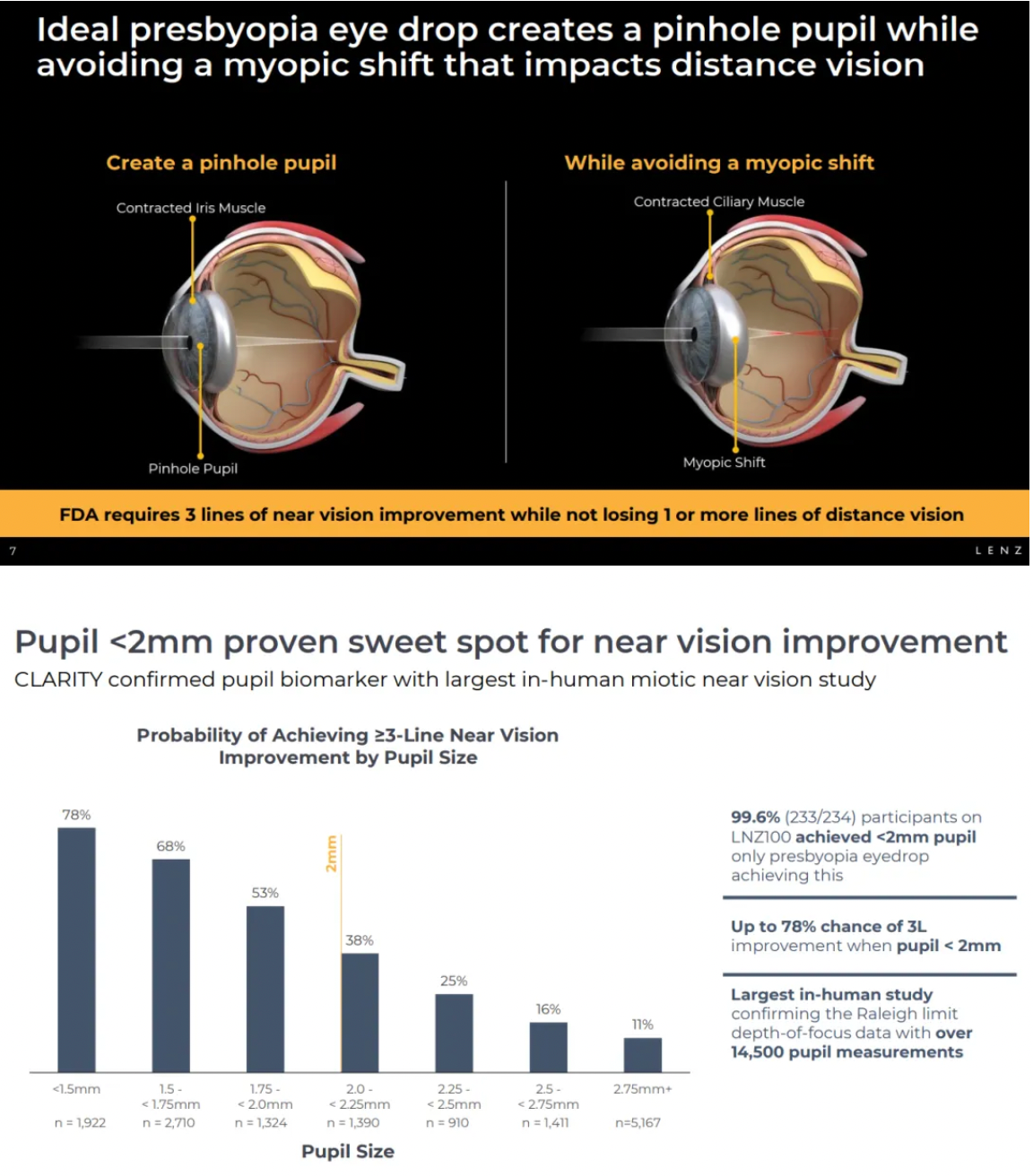
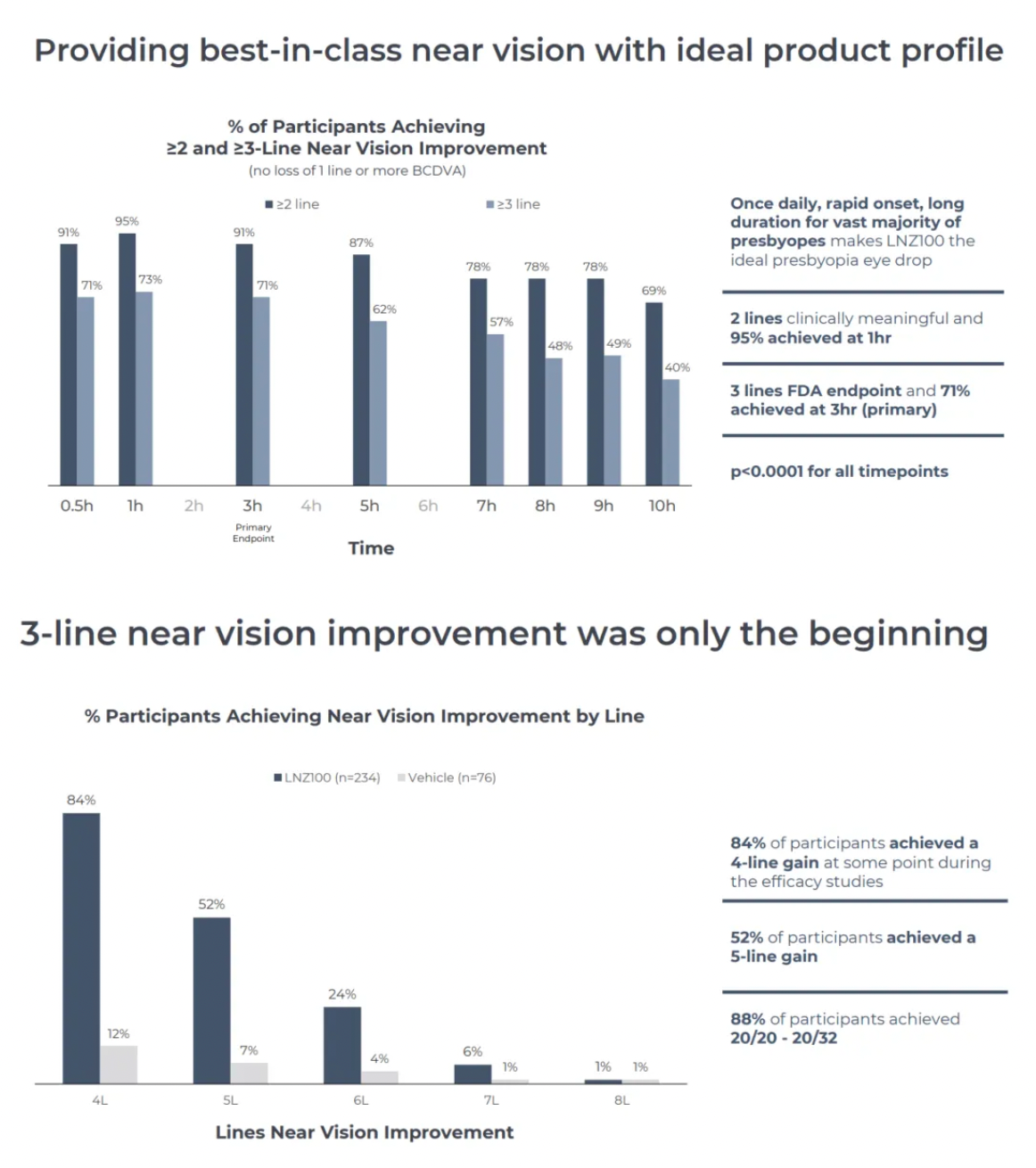
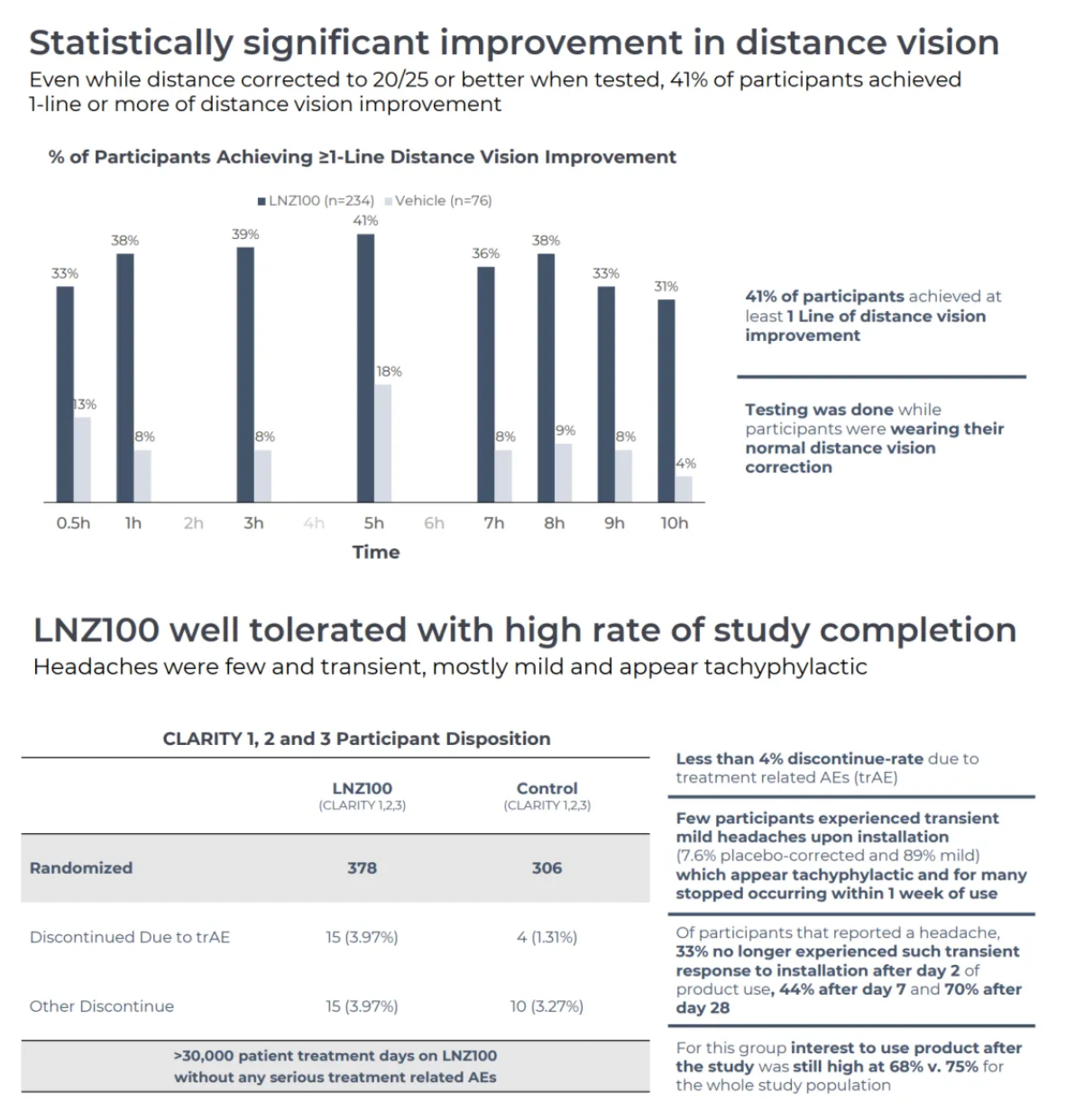
Phase III clinical results for Aceclidine showed rapid onset of action (71% achieved an improvement of three lines or more within 30 minutes), prolonged duration (40% maintained this level of improvement up to 10 hours), effective performance (consistent and reproducible near vision improvement throughout the four-week study periods of CLARITY 1 and 2), and good tolerability (no treatment-related serious adverse events were observed across over 30,000 treatment days in all three CLARITY trials).
The FDA will have 60 days to review the NDA submission materials to determine if they are complete and suitable for further review.
Notably, following the completion of its Series B financing round of approximately $83.5 million in 2023, LENZ Therapeutics announced in March this year that it had completed a merger with Graphite Bio. The merged entity will operate under the name LENZ Therapeutics and is set to begin trading on the NASDAQ on March 22, 2024, under the ticker symbol “LENZ.” Recently, LENZ also secured a $30 million investment from Ridgeback Capital.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!