Halia Therapeutics Advances Phase 2 Trial for HT-6184 in LR-MDS
Halia Therapeutics, an innovative biopharmaceutical firm focused on treatments for chronic inflammation and associated conditions, has revealed encouraging topline data that has led to the progression into the second phase of its Phase 2 clinical trial. This trial is assessing HT-6184, an oral first-in-class allosteric NEK7/NLRP3 inflammasome inhibitor, for use in patients with lower-risk myelodysplastic syndromes (LR-MDS).
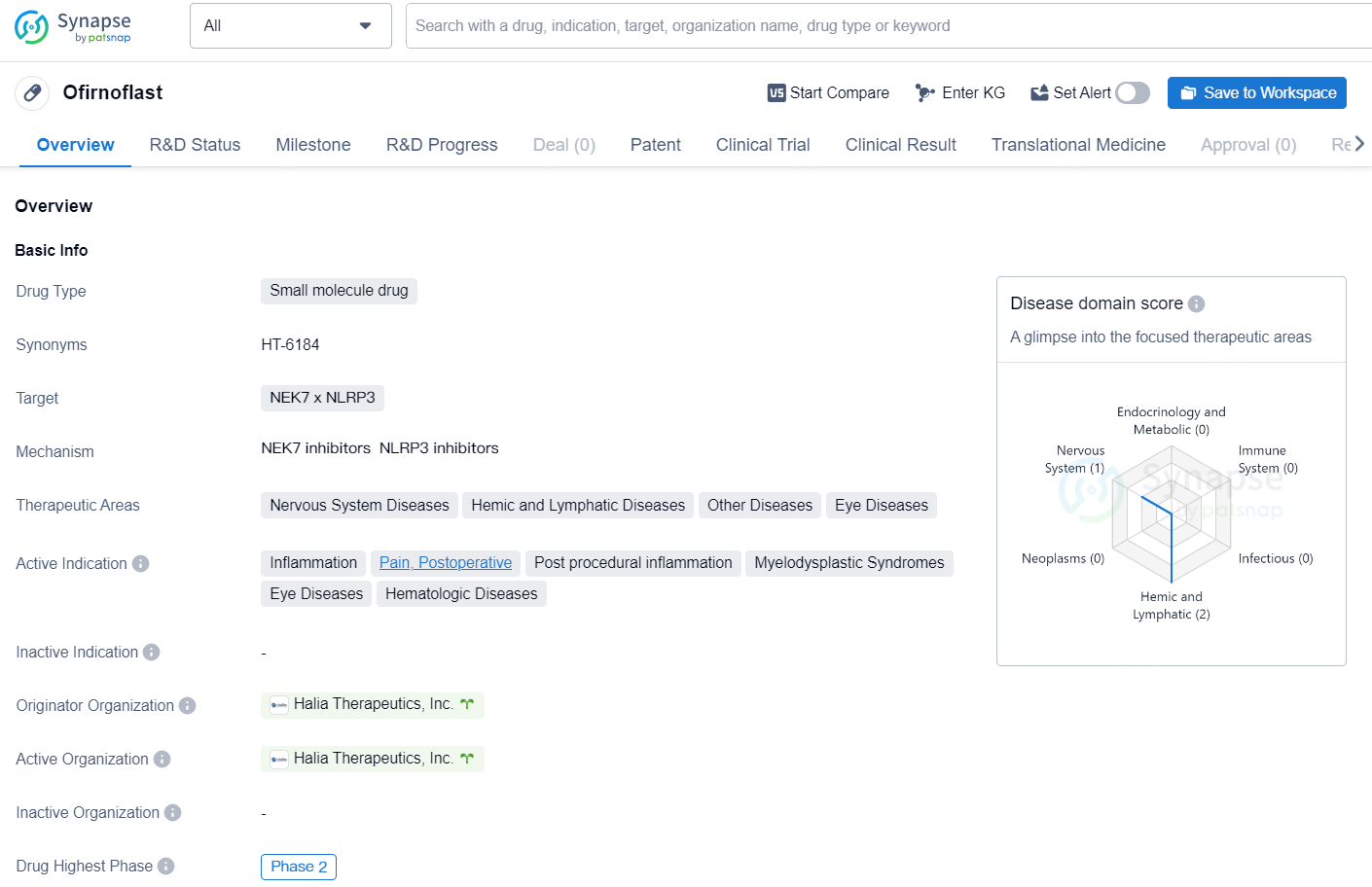
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The initial group of 18 patients diagnosed with LR-MDS showed a hematological improvement-erythroid (HI-E) response after 16 weeks of treatment with HT-6184 monotherapy, surpassing the threshold of three responders that was established beforehand. Having reached this significant endpoint, the trial is now moving forward to its second phase, which will involve enrolling an additional 8-10 patients to further assess the efficacy of HT-6184.
“The notable rate of erythroid response observed with HT-6184 underscores the critical roles of the NLRP3 inflammasome and myddosome pathways as key factors in the ineffective hematopoiesis seen in MDS, indicating a promising avenue for a safe and effective oral treatment for LR-MDS patients,” noted Alan List, MD, a member of the Halia Scientific Advisory Board.
"This study marks an important advancement in meeting the unmet needs of LR-MDS patients," stated Dr. David J. Bearss, Ph.D., President and CEO of Halia Therapeutics. "By targeting the inflammatory signaling that contributes to this condition, HT-6184 seeks to enhance treatment results and improve the quality of life for patients with limited treatment choices."
The Phase 2 study employs a Simon’s minimax two-stage approach to assess both the safety and effectiveness of HT-6184 in as many as 40 patients at various clinical sites throughout India. The primary objectives include evaluating hematologic improvements such as dependency on transfusions and alterations in hemoglobin levels. Secondary objectives focus on HT-6184’s influence on biomarkers of inflammasome activity in MDS and the magnitude of somatic gene mutation clones, providing a thorough assessment of its therapeutic capabilities.
Completion of the trial is anticipated by the end of the second quarter of 2025. The findings from this study are expected to yield critical data that will facilitate the continued clinical development of HT-6184 and its future regulatory applications.
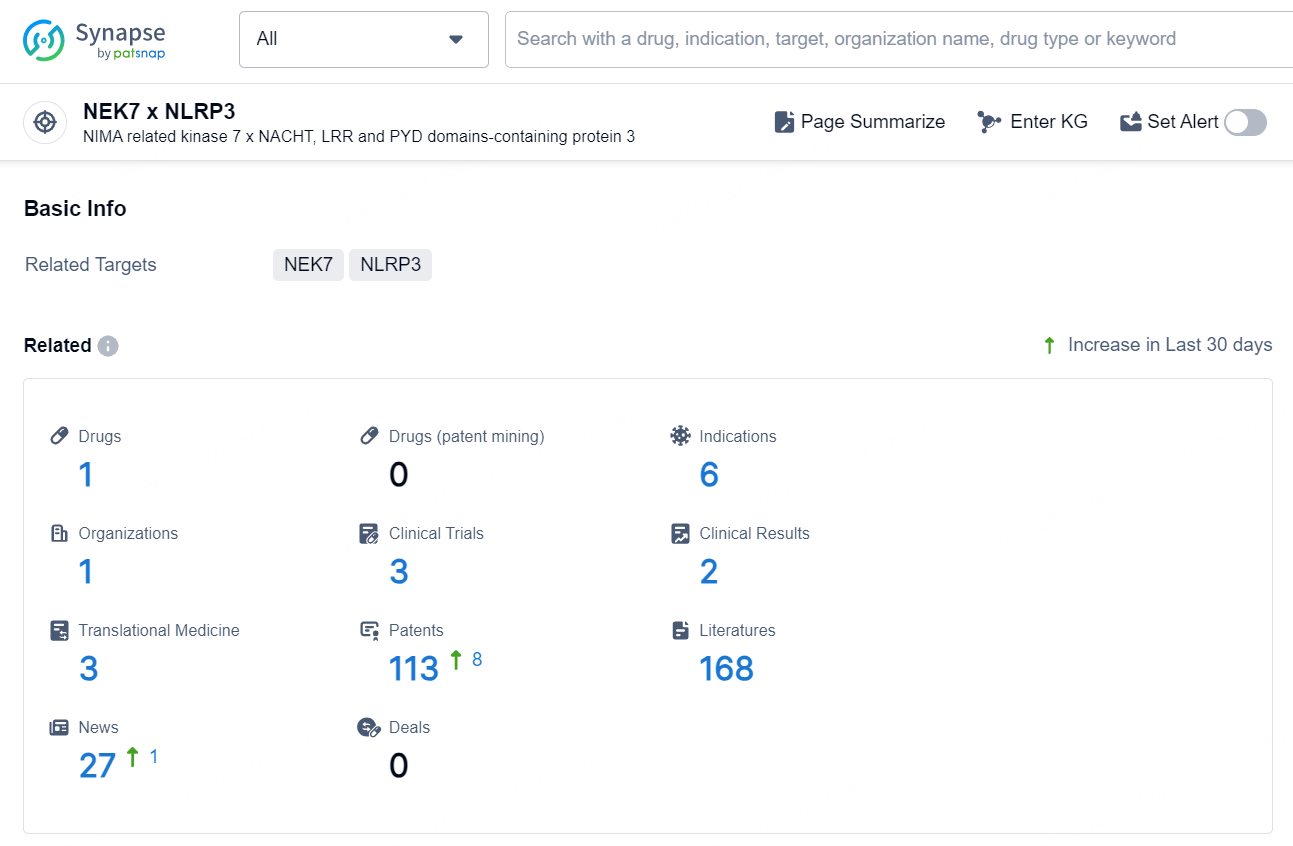
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 17, 2024, there are 1 investigational drug for the NEK7 x NLRP3 target, including 6 indications, 1 R&D institution involved, with related clinical trials reaching 3, and as many as 113 patents.
Ofirnoflast is a small molecule drug developed by Halia Therapeutics, Inc. that targets NEK7 x NLRP3 and is currently in Phase 2 of clinical development. The drug is being developed for the treatment of various therapeutic areas including Nervous System Diseases, Hemic and Lymphatic Diseases, Other Diseases, and Eye Diseases.