Leucid Bio receives MHRA approval to clinically test LEU011, a Lateral NKG2D CAR-T Cell treatment
Leucid Bio, an independent biotech firm diligently striving to revolutionize Chimeric Antigen Receptor T-cell therapies leveraging its exclusive Lateral CAR platform, made an announcement today that it has been granted the CTA by U.K. MHRA. They can now initiate the Phase 1/2 AERIAL clinical trials to assess the safety and effectiveness of LEU011 in treating adults diagnosed with relapsed or resistant solid tumours.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the month of September 2022, LEU011 was bestowed with the title of Innovative Medicine, or Innovation Passport, under the Innovative Licensing and Access Pathway (ILAP) from the MHRA. This award is meant to indicate the medicine's potential for treating solid tumours that express NKG2D ligands. The ILAP was put into practice in 2021 as a method to foster unique approaches towards the creation of drugs while ensuring safety, speed, and efficiency, ultimately leading to wider patient access to medicines.
"The approval by MHRA and the recognition from ILAP of LEU011 emphasises the great promise of our own lateral CAR T cell therapy, LEU011, specifically in treating solid tumours," expressed Filippo Petti, CEO of Leucid Bio.
"Our work on LEU011 is testament to our commitment to pushing the envelope in cell therapy investigations and signifies our unwavering focus on devising fresh, long-term cancer treatments for patients who currently have limited choices." Additional comments were made by Filippo Petti.
Dr. John Maher, CSO at Leucid Bio, mentioned, "The AERIAL trial, sparked by our optimistic preclinical results for LEU011, seeks to evaluate the expansive potential of this creative cellular therapy against various solid tumours expressing NKG2D ligands. We eagerly anticipate launching the AERIAL examination soon and are excited about releasing the initial human trial data for LEU011 by 2024.”
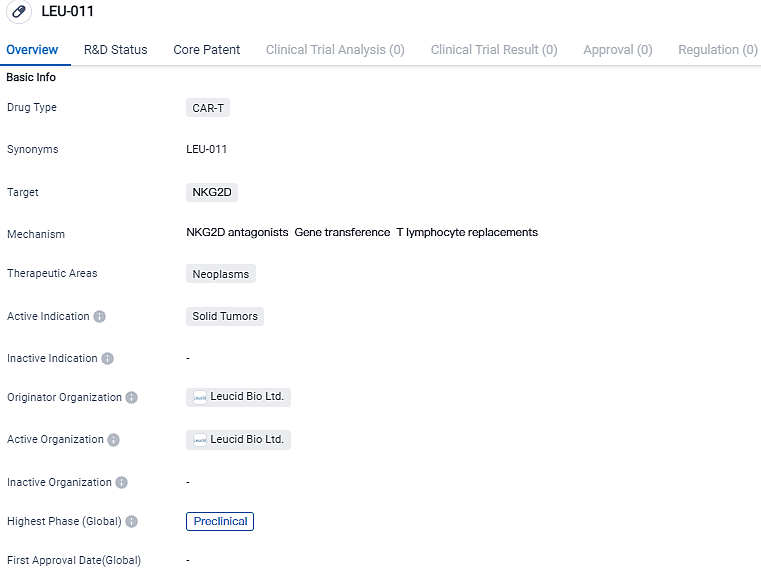
LEU011 is a novel, self-derived, lateral CAR T cell therapy that targets NKG2D ligands. The NKG2D receptor facilitates the immune response against one or more of the eight human NKG2D ligands found on transformed, infected, or compromised cells. There is substantial potential for LEU011 to treat diverse types of cancer, given that NKG2D ligands are said to be present in over 80% of human tumours.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 28, 2023, there are 27 investigational drugs for the NKG2D target, including 29 indications,24 R&D institutions involved, with related clinical trials reaching 57,and as many as 9430 patents.
Leucid Bio Ltd. is spearheading the development of LEU-011, a CAR-T therapy for addressing solid tumor conditions. This proposed treatment aims at the NKG2D receptor, and is presently in the preclinical test phase. Despite exhibiting promising prospects in the biomedical sector, it necessitates additional investigation and evaluation to establish its efficacy and safety.