Advances in Clinical Research on PARP1 inhibitors
PARP, or Poly (ADP-ribose) polymerase, is a type of single-strand DNA repair enzyme that plays a critical role in DNA damage repair and maintaining genome stability. The PARP family includes several members, amongst which only PARP1, PARP2 and PARP3 are involved in DNA repair. PARP1 is the most prominent member of the PARP family, accounting for 80%-90% of the enzyme activity, and is a key player in DNA damage repair.
BRCA1/2 are two suppressor genes discovered early on, playing a significant role in the homologous recombination repair (HR) process. When DNA breaks occur, proteins expressed by BRCA1 and BRCA2 bind with recombination enzyme RAD51 to complete the DNA repair process. Research has found that mutations of the BRCA gene limit DNA repair function and lead to the development of cancer, particularly breast and ovarian cancer.
In 2005, it was first confirmed that a "synthetic lethal" effect exists between PARP inhibitors and Breast Cancer Susceptibility Gene (BRCA) 1/2 mutations. When PARP function is inhibited, it often leads to single-strand breaks developing into double-strand breaks. In cells where the homologous recombination repair pathway is normal, the aforementioned DNA double-strand breaks are immediately repaired. However, in cancer cells lacking homologous recombination repair, such as breast cancer cells carrying BRCA1/2 mutations, the loss of PARP1 function lacks compensation mechanisms. The cancer cells cannot timely repair DNA damage, eventually leading to cell death, a mechanism known as synthetic lethality. Based on this principle, PARP inhibitors can induce the death of cancer cells carrying germline BRCA mutations.
PARP1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 51 PARP1 drugs worldwide, from 91 organizations, covering 107 indications, and conducting 1225 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.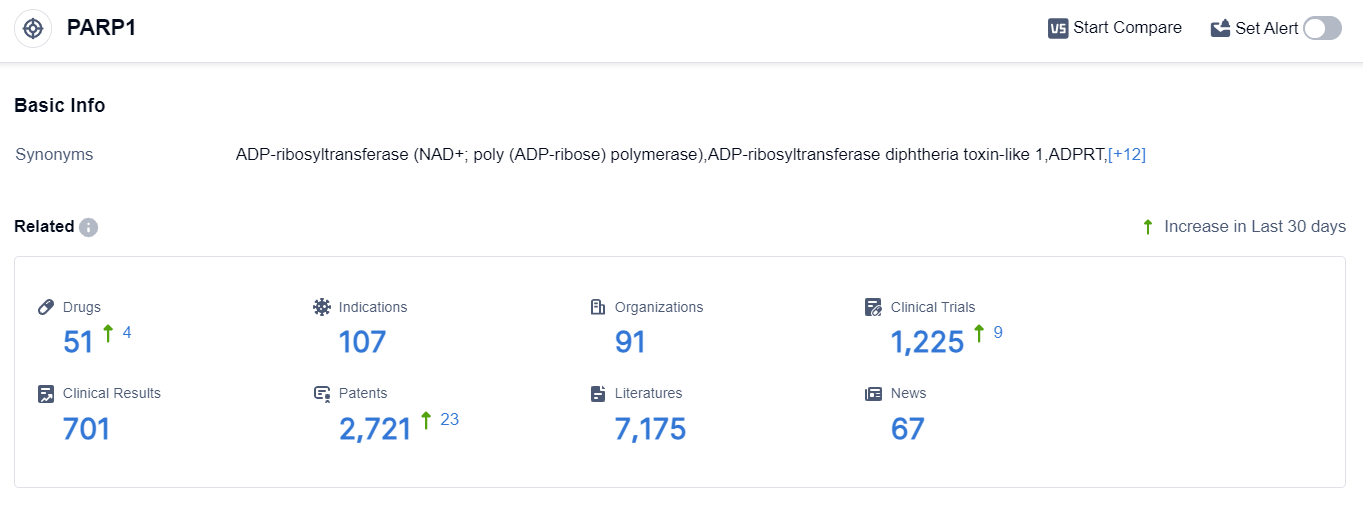
The analysis of the current competitive landscape of target PARP1 reveals that multiple companies are actively involved in the development of drugs targeting this target. AstraZeneca PLC, Pfizer Inc., GSK Plc, and BeiGene Ltd. are leading in terms of the highest stage of development.
The indications for the approved drugs under target PARP1 include various types of cancer, such as ovarian cancer, prostate cancer, breast cancer, and pancreatic cancer. Small molecule drugs are progressing rapidly, indicating intense competition in this area.
The United States, European Union, and China are the key countries/locations driving the development of drugs targeting PARP1. China, in particular, has shown significant progress in this field. Overall, the target PARP1 presents a competitive landscape with promising future development potential in the pharmaceutical industry.
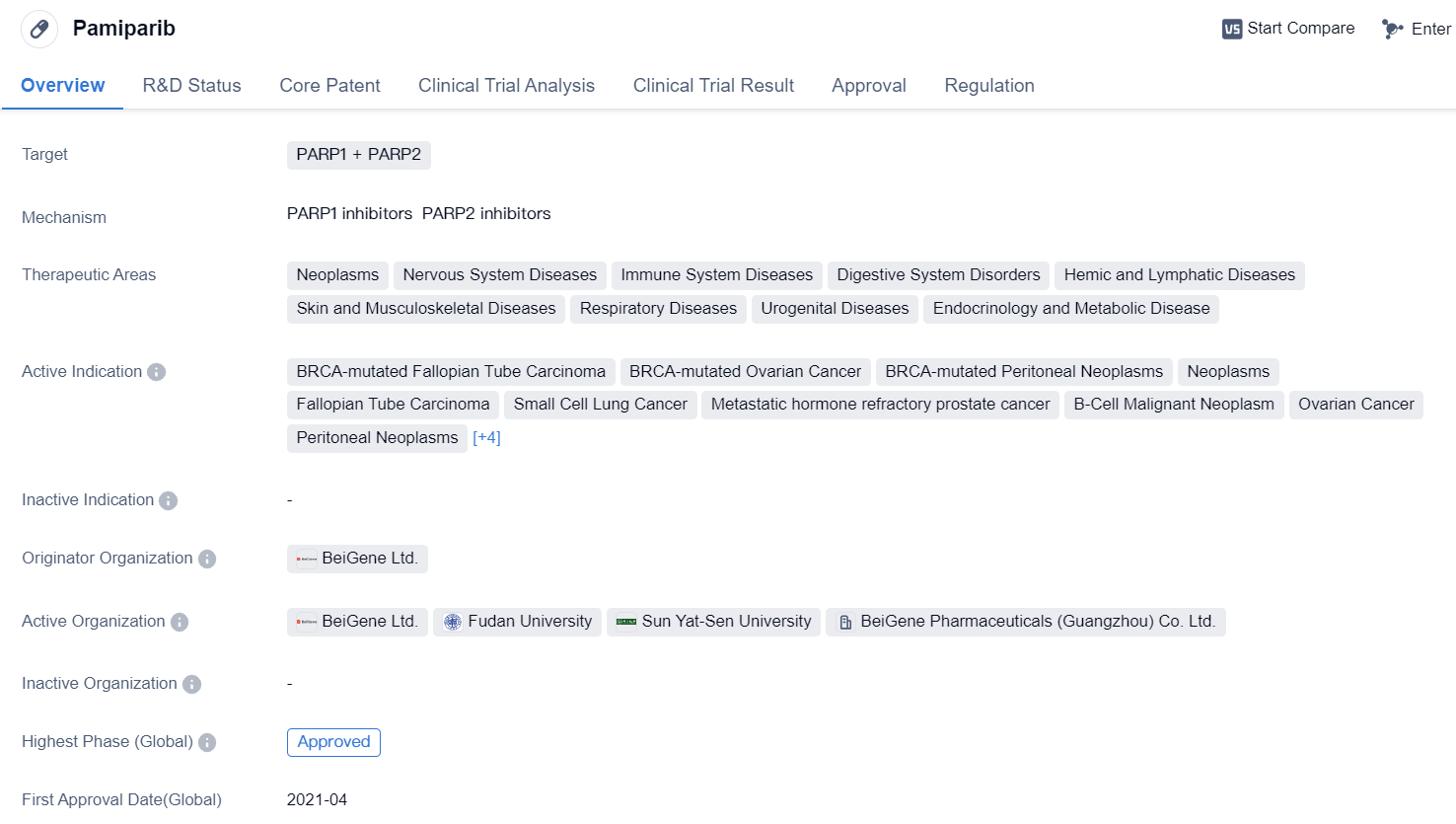
PARP1 and PARP2 Dual Inhibitors: Pamiparib
Pamiparib is a small molecule drug that targets PARP1 and PARP2. It has been approved for use in various therapeutic areas including neoplasms, nervous system diseases, immune system diseases, digestive system disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, and endocrinology and metabolic diseases.
The drug has shown efficacy in treating several active indications, including BRCA-mutated Fallopian Tube Carcinoma, BRCA-mutated Ovarian Cancer, BRCA-mutated Peritoneal Neoplasms, Neoplasms, Fallopian Tube Carcinoma, Small Cell Lung Cancer, Metastatic hormone refractory prostate cancer, B-Cell Malignant Neoplasm, Ovarian Cancer, Peritoneal Neoplasms, Stomach Cancer, Triple Negative Breast Cancer, Glioblastoma, and Solid Tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Pamiparib was developed by BeiGene Ltd., an originator organization in the pharmaceutical industry. It has received approval in both global and Chinese markets, with the highest phase of approval being "Approved" in both regions. The drug obtained its first approval in China in April 2021.
In terms of regulation, Pamiparib has undergone priority review, conditional marketing approval, and is part of a special review project. These regulatory designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
Overall, Pamiparib is a small molecule drug that targets PARP1 and PARP2 and has shown promise in treating various diseases across multiple therapeutic areas. Its approval in both global and Chinese markets, along with its regulatory designations, indicate its potential as a valuable treatment option.
High selectivity PARP1 inhibitor: AZD-5305
AZD-5305 is a small molecule drug that targets PARP1, an enzyme involved in DNA repair. It is being developed by AstraZeneca PLC, a leading pharmaceutical company. The drug is currently in Phase 2 of clinical trials, both globally and in China.
AZD-5305 has shown potential in treating various types of cancers, particularly neoplasms, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, and urogenital diseases. The active indications for this drug include colorectal cancer, endometrial carcinoma, metastatic hormone refractory prostate cancer, ovarian cancer, stomach cancer, prostatic cancer metastatic, breast cancer, pancreatic cancer, solid tumors, and prostatic cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Colorectal cancer is a type of cancer that affects the colon or rectum, while endometrial carcinoma refers to cancer that develops in the lining of the uterus. Metastatic hormone refractory prostate cancer is a form of prostate cancer that has spread to other parts of the body and is resistant to hormone therapy. Ovarian cancer affects the ovaries, while stomach cancer affects the stomach lining. Prostatic cancer metastatic refers to prostate cancer that has spread to other organs. Breast cancer is a type of cancer that develops in breast cells, and pancreatic cancer affects the pancreas. Solid tumors refer to abnormal growths of cells in various tissues, and prostatic cancer specifically targets the prostate gland.
The development of AZD-5305 is significant as it offers potential treatment options for a wide range of cancers. By targeting PARP1, the drug aims to disrupt DNA repair mechanisms in cancer cells, leading to their death or reduced growth. This approach is particularly promising in cancers where DNA repair mechanisms are already compromised.
The fact that AZD-5305 has reached Phase 2 of clinical trials indicates that it has shown promising results in early studies, demonstrating its safety and efficacy to a certain extent. Further research and development will be necessary to determine the drug's full potential and its effectiveness in treating the mentioned indications.
In conclusion, AZD-5305 is a small molecule drug developed by AstraZeneca PLC that targets PARP1. It is currently in Phase 2 of clinical trials globally and in China. The drug shows potential in treating various types of cancers, including colorectal cancer, endometrial carcinoma, metastatic hormone refractory prostate cancer, ovarian cancer, stomach cancer, prostatic cancer metastatic, breast cancer, pancreatic cancer, solid tumors, and prostatic cancer. Further research is needed to fully understand the drug's efficacy and safety profile.