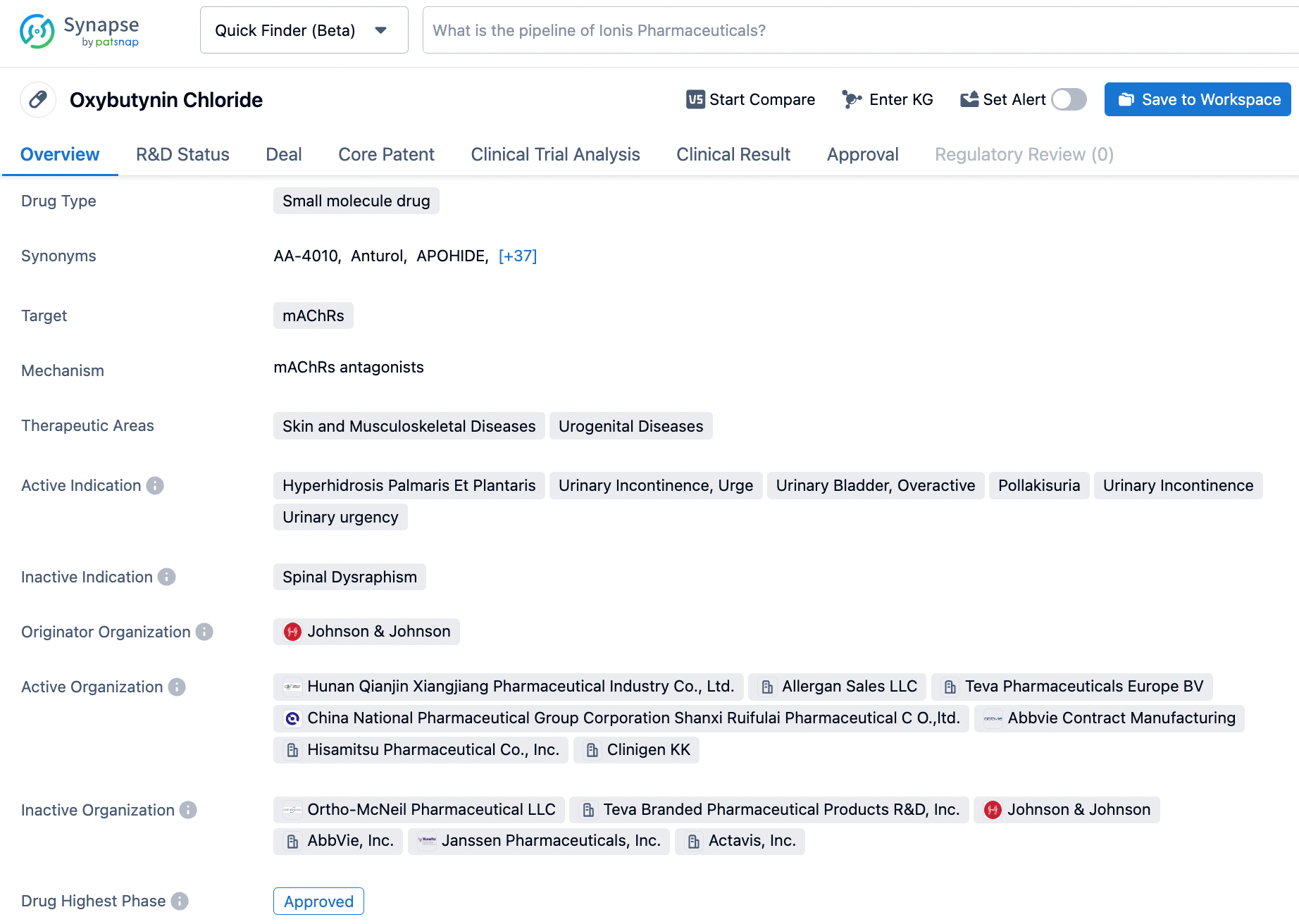
Maximize Your Synapse Use: Your Guide to Searching Oxybutynin
Oxybutynin, marketed as DITROPAN®, is a medication approved by the US FDA in 1975 for the treatment of overactive bladder. Developed by Janssen, the drug works as an antagonist of acetylcholine at postganglionic muscarinic receptors, causing relaxation of the smooth muscle in the bladder. It is a racemic mixture of R- and S-isomers, with the antimuscarinic activity mainly residing in the Risomer. Additionally, the active metabolite, N-desethyloxybutynin, has similar pharmacological effects on human detrusor muscle as oxybutynin, as observed in in vitro studies. Overall, Oxybutynin Chloride offers relief to those experiencing urinary urgency, frequency, and incontinence. Click on the image below to begin the exploration journey of Oxybutynin through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!