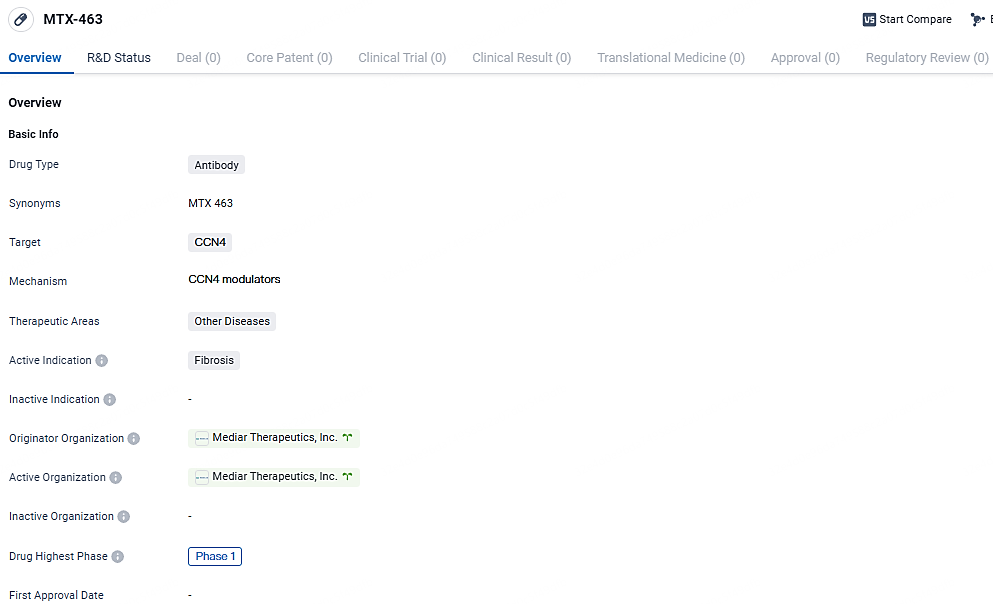
Mediar Begins Phase 1 Study of MTX-463 and Establishes Clinical Advisory Committee
Mediar Therapeutics, Inc., a biotech firm focused on developing a unique range of treatments designed to stop the progression of fibrosis, recently declared that the initial group of volunteers received their doses in a Phase 1 study. This occurred after the FDA approved its application to investigate a new drug. The trial aims to assess the safety and tolerability of MTX-463.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
MTX-463 is an innovative human IgG1 antibody crafted to inhibit the fibrotic signaling caused by WISP-1, aiming to treat idiopathic pulmonary fibrosis (IPF) and other related fibrotic conditions. The initial Phase 1 trial of MTX-463 is structured to evaluate its safety across various dosage levels, its tolerability, and its pharmacokinetic properties. Additionally, the study aims to verify the effectiveness of MTX-463 in targeting WISP-1. The ongoing trial includes healthy subjects and features both staggered single ascending dose and multiple ascending dose groups.
"Reaching the clinical stage with our pioneering portfolio marks a significant achievement for Mediar, fulfilling a vision laid out by our founders from Mass General Brigham and pursued vigorously by our team," declared Dr. Rahul Ballal, CEO of Mediar. "MTX-463 is one of two treatments inaugurating clinical trials this year, and we are eager to start a second Phase 1 trial involving our groundbreaking anti-EphrinB2 agent, MTX-474, in the third quarter."
As MTX-463 and MTX-474 move into human clinical trials, Mediar is supported by its clinical advisory board, which includes globally acclaimed experts in fibrosis from fields such as pulmonology, rheumatology, hepatology, and regulatory affairs. "Our Clinical Advisory Board's formation brings together extensive expertise and knowledge, enhancing the development of our clinical programs as we tackle the critical need in fibrosis treatment," remarked Dr. Jeff Bornstein, Mediar's Chief Medical Officer.
MTX-463 represents a novel approach in anti-fibrotic therapy as a human IgG1 antibody targeting the WNT1-inducible signaling pathway protein-1 (WISP-1), a key player in fibrosis. WISP-1, a protein implicated in the enhancement of fibrotic processes, can be measured in human blood and its levels associate with disease severity. Preliminary research shows that MTX-463 effectively interrupts WISP-1-driven fibrotic signaling linked to various fibrotic diseases, and it has substantially reduced fibrosis in vitro and in animal models.
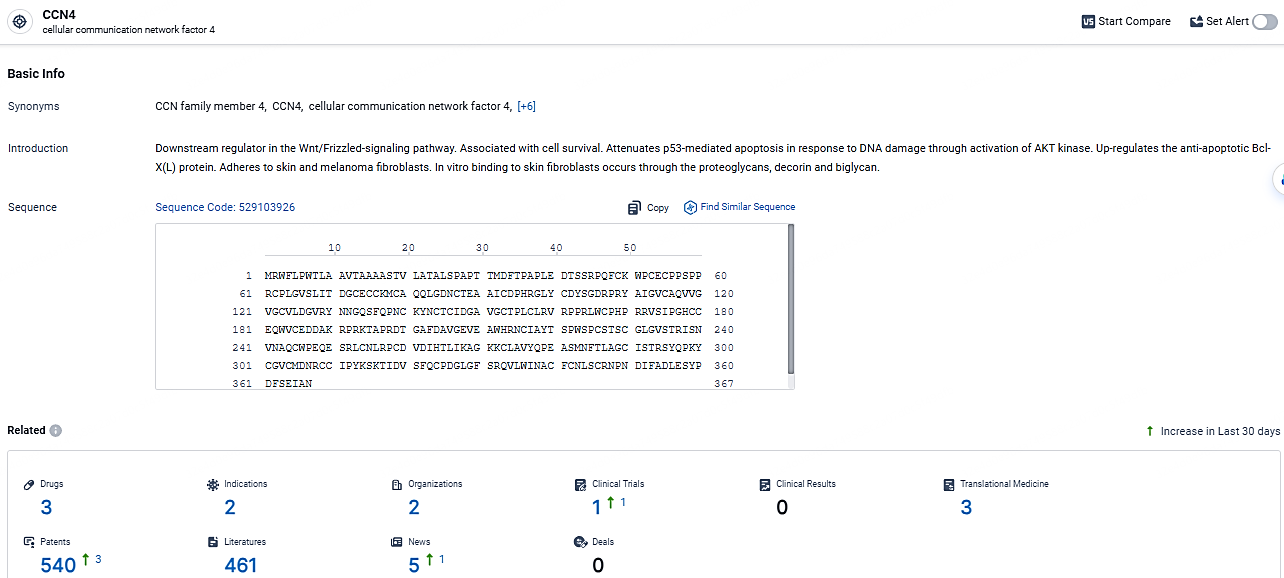
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 7, 2024, there are 3 investigational drugs for the CCN4 targets, including 2 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 540 patents.
MTX-463 targets CCN4 and is being developed for the treatment of fibrosis. Currently in Phase 1 of clinical development, the drug aims to inhibit CCN4 activity and potentially address fibrosis-related complications. The therapeutic areas of MTX-463 extend beyond fibrosis, but further details regarding specific diseases or conditions are not provided.