Merck Unveils Early Results for RSV Antibody Clesrovimab in Infants
Merck, referred to as MSD outside the U.S. and Canada, reported favorable topline outcomes from its Phase 2b/3 clinical trial investigating clesrovimab (MK-1654). This is Merck’s experimental prophylactic monoclonal antibody aimed at safeguarding infants against respiratory syncytial virus (RSV) disease. The study demonstrated that clesrovimab achieved its main safety and effectiveness goals, such as decreasing incidents of medically attended lower respiratory tract infections due to RSV up to Day 150.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 "RSV is extremely contagious and can cause airway inflammation in infants, resulting in breathing difficulties. As a prevalent illness worldwide, RSV is the main cause for hospitalizing healthy infants," stated Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines at Global Clinical Development, Merck Research Laboratories. "We are optimistic about these results and look forward to cooperating with regulators to offer a new solution to mitigate the impact of RSV on infants and their families."
"RSV is extremely contagious and can cause airway inflammation in infants, resulting in breathing difficulties. As a prevalent illness worldwide, RSV is the main cause for hospitalizing healthy infants," stated Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines at Global Clinical Development, Merck Research Laboratories. "We are optimistic about these results and look forward to cooperating with regulators to offer a new solution to mitigate the impact of RSV on infants and their families."
MK-1654-004 is a Phase 2b/3 double-blind, randomized, placebo-controlled study designed to assess the safety and efficacy of clesrovimab in healthy preterm and full-term infants. Participants were assigned randomly to receive either a single dose of clesrovimab or a placebo. The primary endpoints include the rate of participants with RSV-associated medically attended lower respiratory infections from Day 1 to Day 150 compared to placebo and safety.
Safety assessments included the percentage of participants experiencing any injection-related adverse events, AEs of special interest, solicited systemic AEs, or serious adverse events.
Clesrovimab (MK-1654) is an investigational extended half-life monoclonal antibody developed for passive immunization to prevent RSV-associated medically attended lower respiratory infections. Clesrovimab is being examined in infants (both pre-term and full-term) to deliver rapid, long-lasting protection through their first RSV season with a single, fixed-dose administration.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
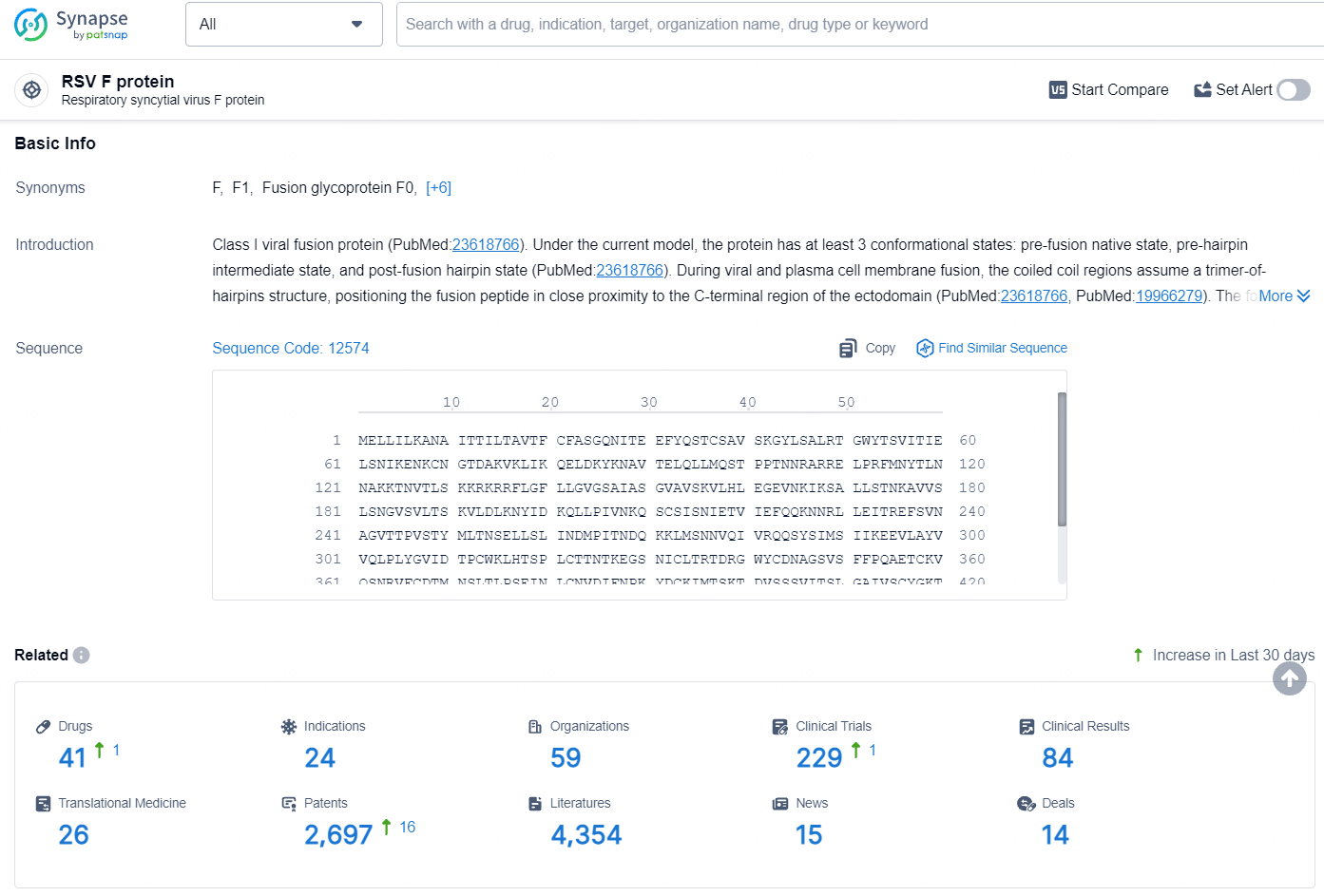
According to the data provided by the Synapse Database, As of July 25, 2024, there are 41 investigational drugs for the RSV protein targets, including 24 indications, 59 R&D institutions involved, with related clinical trials reaching 229, and as many as 2697 patents.
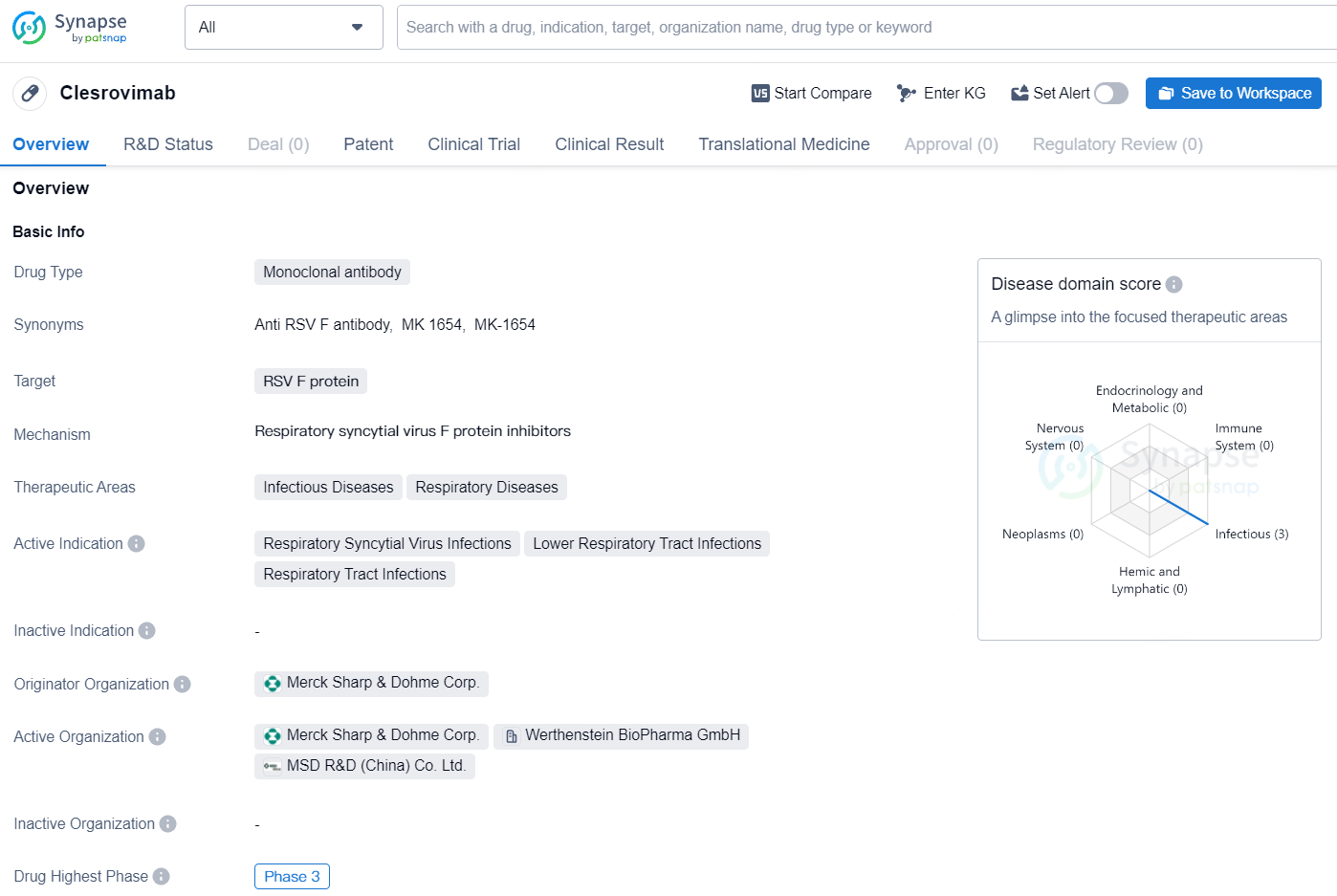
Clesrovimab is a monoclonal antibody drug targeting the RSV F protein, which is being developed by Merck Sharp & Dohme Corp. The drug falls under the therapeutic areas of Infectious Diseases and Respiratory Diseases, with active indications for Respiratory Syncytial Virus Infections, Lower Respiratory Tract Infections, and Respiratory Tract Infections. As per the latest available information, Clesrovimab has reached the highest phase of development, which is Phase 3, on a global scale. In China as well, the drug has obtained the highest phase of development, which is also Phase 3.