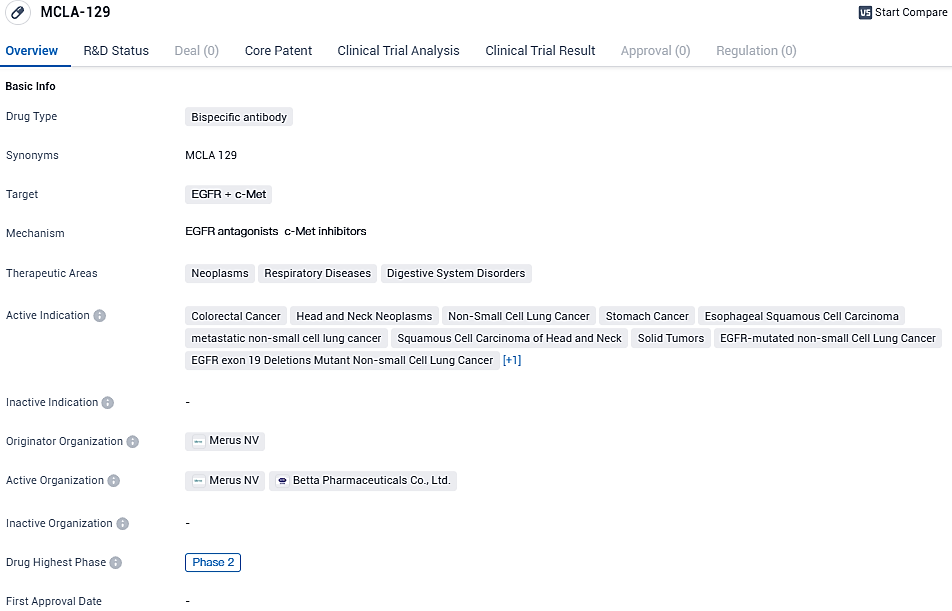
Merus Showcases Preliminary Results for MCLA-129 at the 2023 ESMO Asia Congress
Merus N.V., an enterprise functioning in the clinical-phase and focusing on crafting cutting-edge, fully-integrated multispecific antibodies named Biclonics® and Triclonics®, has revealed fresh interim clinical findings on their candidate molecule MCLA-129. This information includes the latest results from current broader participant groups suffering from non-small cell lung cancer and those who have undergone prior treatments for head and neck squamous cell carcinoma. These findings were shared with the medical community at the 2023 ESMO Asia Congress.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
MCLA-129 has shown significant efficacy in treating EGFRm-mutant NSCLC, and our team is gearing up for targeted investments to assess the synergistic effects of MCLA-129 when used alongside standard chemotherapy treatments. We're targeting an early 2024 timeline to commence these evaluations," stated Bill Lundberg M.D., the CEO and President of Merus.
Dr. Lundberg continued, "Our financial status is robust, and we are vigilant in managing our resources wisely to sustain our financial health. With this in mind, we have outlined plans to kick-off a phase 3 study of petosemtamab for 2L+ HNSCC around the mid-2024 timeframe."
The disseminated figures pertain to three specific patient groups within the ongoing study assessing MCLA-129 in tandem with osimertinib—an advanced EGFR TKI—in EGFR mutant NSCLC patients who have not previously undergone treatment and in those with EGFR mutation-positive NSCLC showing progression post-osimertinib treatment. The data also cover investigations into MCLA-129 as a single-agent regimen for HNSCC patients who have received prior therapies.
MCLA-129, a Biclonics® therapeutic agent enhanced for antibody-dependent cellular cytotoxicity, is strategically engineered to impede the EGFR and c-MET signaling routes implicated in various solid neoplasms. Its design integrates dual mechanisms: one that impedes proliferative and survival signaling to curb tumor growth, and another that mobilizes and amplifies immune effector cells to eradicate cancer cells.
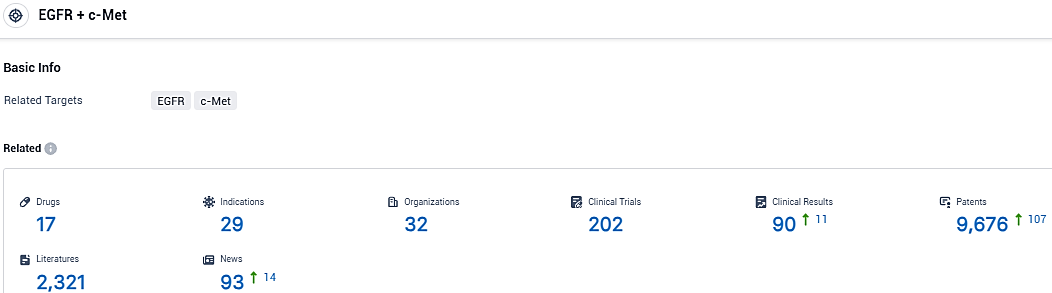
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 12, 2023, there are 17 investigational drugs for the EGFR and c-Met target, including 29 indications, 32 R&D institutions involved, with related clinical trials reaching 202, and as many as 9676 patents.
The development of MCLA-129 is an important advancement in the field of biomedicine, as it offers a potential treatment option for multiple types of cancer and respiratory diseases. The drug's ability to target both EGFR and c-Met may provide a more comprehensive approach to inhibiting cancer cell growth and metastasis.