European regulators have approved UCB's ZILBRYSQ® (zilucoplan) for adult generalized Myasthenia Gravis treatment
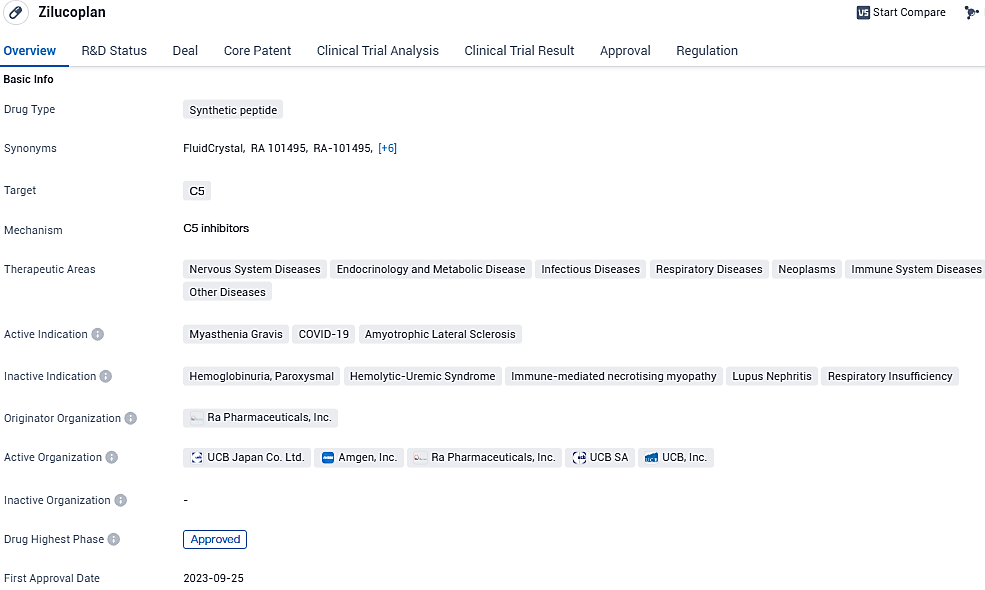
Global biopharma leader UCB has received a green light from the European Commission (EC) to introduce ZILBRYSQ® (zilucoplan) into the market. This approval endorses the drug's use in combination with traditional treatments, specifically aimed at managing adult patients diagnosed with generalized myasthenia gravis who have tested positive for anti-acetylcholine receptor antibodies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Zilucoplan emerges as the inaugural subcutaneous, once-a-day, targeted peptide modulator for gMG, precisely inhibiting the complement component 5 (C5), and stands as the sole C5 modulator sanctioned for self-injection by gMG patients over the age of 18 with AChR antibodies.
Through its precise bimodal action, zilucoplan mitigates damage at the neuromuscular junction caused by the complement system. Advantages of self-injection via the subcutaneous route encompass a reduction in travel time to healthcare facilities, minimized disruption to employment commitments, and an enhancement in patient autonomy.
European endorsement for zilucoplan is underpinned by robust safety and effectiveness outcomes from the RAISE trial, whose findings were detailed in The Lancet Neurology during May 2023. The pivotal study was a multi-site, phase 3, placebo-controlled, double-blind, randomized trial designed to evaluate zilucoplan's safety, efficacy, and tolerability among adult gMG patients positive for anti-acetylcholine receptor antibodies.
Zilucoplan's green light in Europe follows earlier regulatory approvals by the FDA for treating adult patients with gMG who are AChR antibody-positive and by the Japanese Ministry of Health, Labour and Welfare for gMG treatment in adults poorly responsive to steroids or other immunotherapy agents.
The green signal issued by the EC extends across all member countries of the EU and includes the European Economic Area nations of Iceland, Liechtenstein, and Norway. UCB aspires to expedite the delivery of zilucoplan to patients and expects to initiate distribution across Europe starting in the early part of 2024..
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
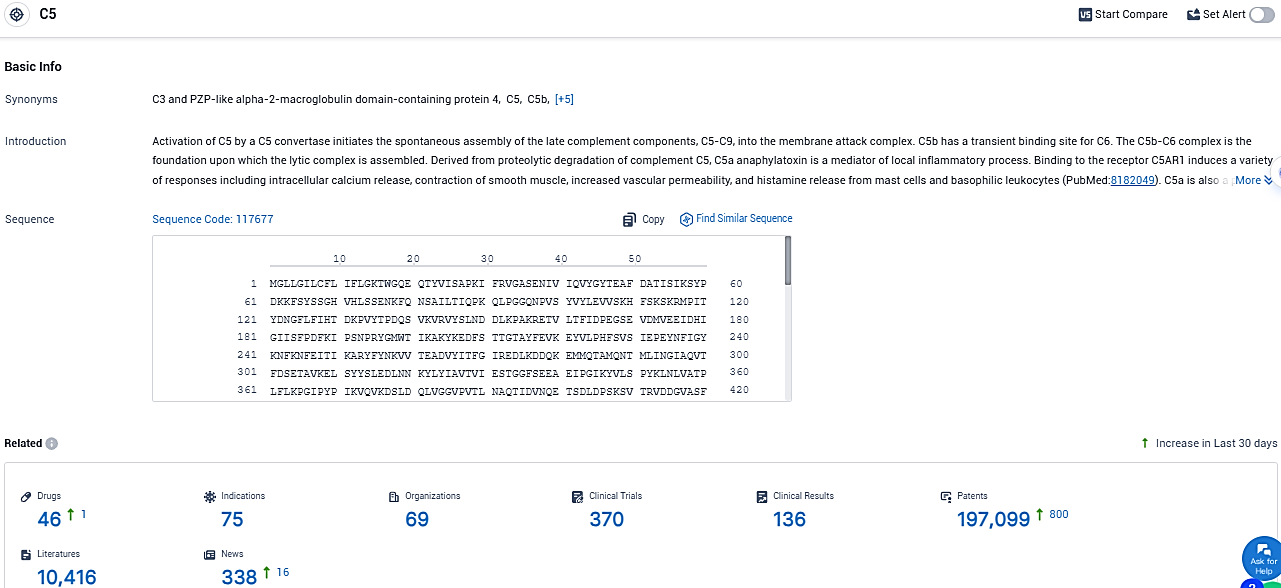
According to the data provided by the Synapse Database, As of December 12, 2023, there are 46 investigational drugs for the C5 target, including 75 indications, 69 R&D institutions involved, with related clinical trials reaching 370, and as many as 197099 patents.
Zilucoplan is currently under review by the Australian Therapeutic Goods Administration and Health Canada for the treatment of adults with gMG. Responses from regulatory agencies to these submissions are expected between H2 2023 and H2 2024. Orphan designation for zilucoplan was granted by the FDA in 2019 for the treatment of myasthenia gravis.