GV20 Therapeutics Partners for Clinical Trial of New Immune Checkpoint Antibody GV20-0251 with KEYTRUDA®
GV20 Therapeutics, a firm specializing in biotechnology at the clinical stage and employing AI, genomics, and disease biology to develop advanced antibody treatments, has declared the initiation of a clinical collaboration and supply contract with Merck.
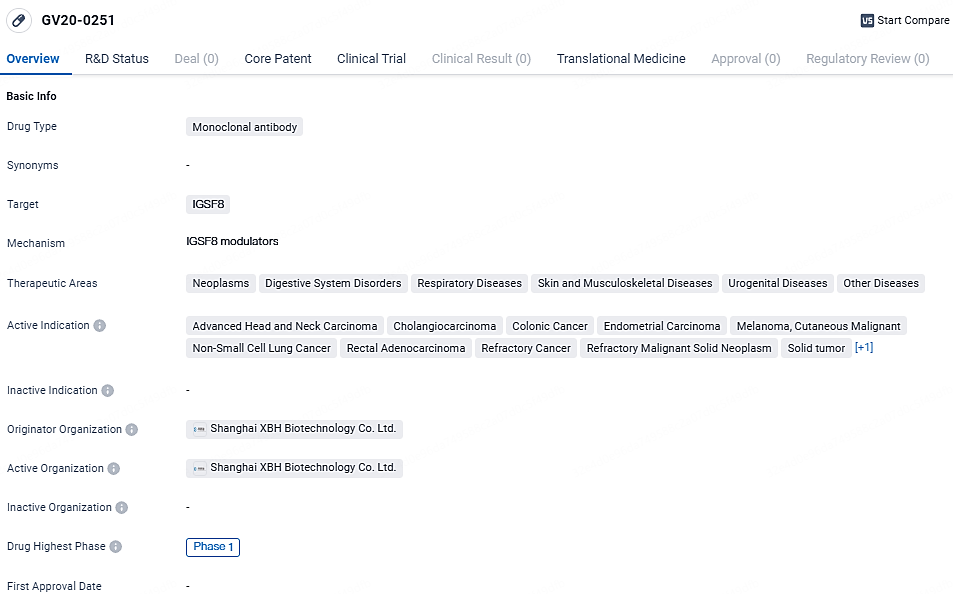
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Under the stipulations of the agreement, GV20 will carry out assessments on its primary research program, GV20-0251. This innovative first-in-class antibody, aimed at the new immune checkpoint IGSF8, will be tested together with Merck’s anti-PD-1 treatment, KEYTRUDA® (pembrolizumab). The tests will be conducted on patients suffering from advanced solid tumors within an existing Phase I trial.
"We're enthusiastic about progressing with the development of GV20-0251 and investigating its efficacy when paired with KEYTRUDA," stated Dr. Shirley Liu, who is the Co-Founder and CEO of GV20 Therapeutics. "IGSF8 represents a breakthrough immune checkpoint that blocks the activity of natural killer cells and dendritic cells. With the blockade lifted through GV20-0251, this antagonistic antibody could emerge as a new foundational tool within the realm of cancer immunotherapy, offering substantial benefits to those afflicted with cancer."
GV20 is progressing with the recruitment for an open-label, multi-center Phase I clinical trial encompassing both dose-escalation and dose-expansion phases. The trial focuses on testing GV20-0251 in patients diagnosed with advanced solid tumors who cannot undergo standard care treatments. The objectives of the study include assessing the safety, tolerability, pharmacokinetic properties, pharmacodynamic responses, and initial anti-tumor effects of GV20-0251, both as a standalone therapy and when used in conjunction with KEYTRUDA® (pembrolizumab).
Preclinical studies by GV20 have shown that IGSF8 antibody blockade delivers robust anti-tumor effects, both as a single treatment and when used alongside anti-PD-1 therapy in various tumor models. At the recent American Association for Cancer Research Annual Meeting 2024, GV20 showcased a presentation titled "IGSF8 is a novel innate immune checkpoint and cancer immunotherapy target" within the framework of the immunology mini-symposium.
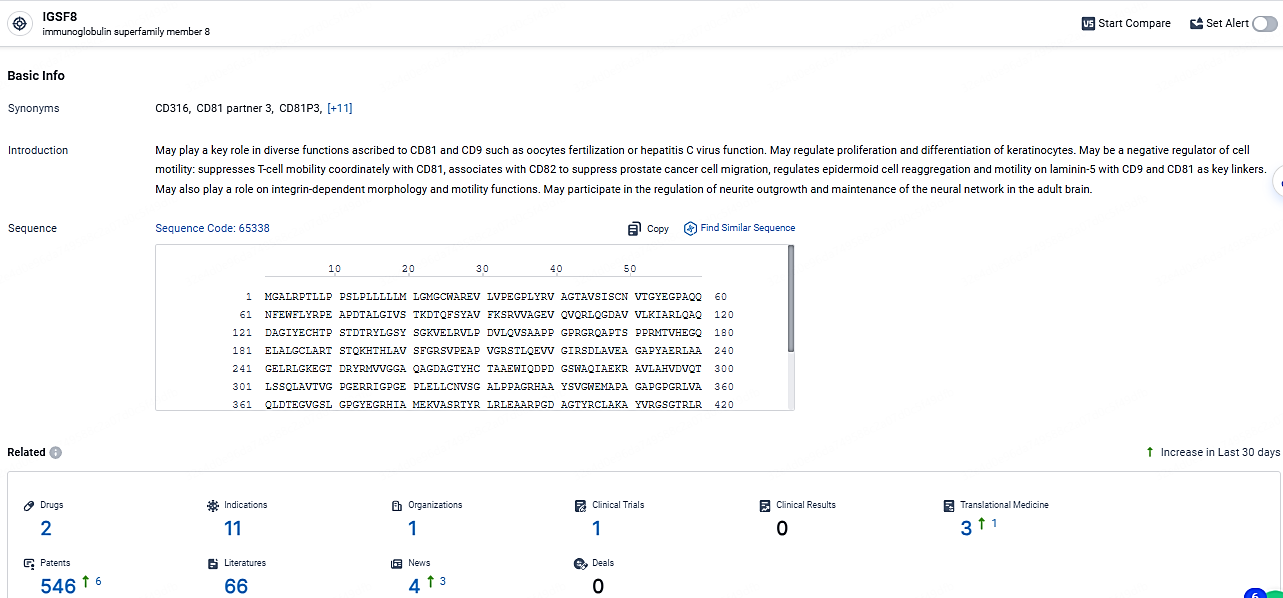
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 2 investigational drugs for the IGSF8 target, including 11 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 546 patents.
GV20-0251 shows promise as a potential therapeutic option for various diseases, particularly in the field of oncology. However, further research and clinical trials are needed to determine its efficacy and safety profile. The development of GV20-0251 represents Shanghai XBH Biotechnology Co. Ltd.'s commitment to advancing biomedicine and addressing unmet medical needs in the pharmaceutical industry.